当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An Artificial Lithium Protective Layer that Enables the Use of Acetonitrile‐Based Electrolytes in Lithium Metal Batteries
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-03-23 , DOI: 10.1002/anie.201801737 Ngoc Duc Trinh 1 , David Lepage 1 , David Aymé-Perrot 2 , Antonella Badia 1 , Mickael Dollé 1 , Dominic Rochefort 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-03-23 , DOI: 10.1002/anie.201801737 Ngoc Duc Trinh 1 , David Lepage 1 , David Aymé-Perrot 2 , Antonella Badia 1 , Mickael Dollé 1 , Dominic Rochefort 1
Affiliation
|
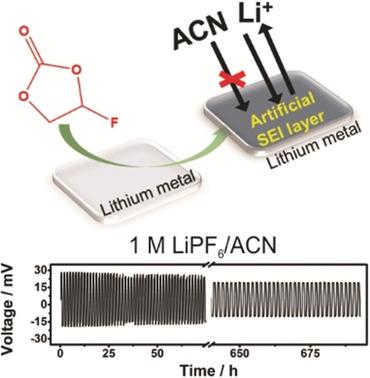
The resurgence of the lithium metal battery requires innovations in technology, including the use of non‐conventional liquid electrolytes. The inherent electrochemical potential of lithium metal (−3.04 V vs. SHE) inevitably limits its use in many solvents, such as acetonitrile, which could provide electrolytes with increased conductivity. The aim of this work is to produce an artificial passivation layer at the lithium metal/electrolyte interface that is electrochemically stable in acetonitrile‐based electrolytes. To produce such a stable interface, the lithium metal was immersed in fluoroethylene carbonate (FEC) to generate a passivation layer via the spontaneous decomposition of the solvent. With this passivation layer, the chemical stability of lithium metal is shown for the first time in 1 m LiPF6 in acetonitrile.
中文翻译:

人造锂保护层,可在锂金属电池中使用乙腈基电解质
锂金属电池的复兴需要技术的创新,包括使用非常规液体电解质。锂金属的固有电化学势(相对于SHE为-3.04 V)不可避免地限制了其在许多溶剂(如乙腈)中的使用,而乙腈可为电解质提供更高的电导率。这项工作的目的是在锂金属/电解质界面处产生一种人工钝化层,该层在乙腈基电解质中具有电化学稳定性。为了产生这种稳定的界面,将锂金属浸入氟代碳酸亚乙酯(FEC)中以通过溶剂的自发分解产生钝化层。借助该钝化层,首次在1 m LiPF中显示了锂金属的化学稳定性 6在乙腈中。
更新日期:2018-03-23
中文翻译:

人造锂保护层,可在锂金属电池中使用乙腈基电解质
锂金属电池的复兴需要技术的创新,包括使用非常规液体电解质。锂金属的固有电化学势(相对于SHE为-3.04 V)不可避免地限制了其在许多溶剂(如乙腈)中的使用,而乙腈可为电解质提供更高的电导率。这项工作的目的是在锂金属/电解质界面处产生一种人工钝化层,该层在乙腈基电解质中具有电化学稳定性。为了产生这种稳定的界面,将锂金属浸入氟代碳酸亚乙酯(FEC)中以通过溶剂的自发分解产生钝化层。借助该钝化层,首次在1 m LiPF中显示了锂金属的化学稳定性 6在乙腈中。



























 京公网安备 11010802027423号
京公网安备 11010802027423号