当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The N‐Terminal Domain of the Pullulanase from Anoxybacillus sp. WB42 Modulates Enzyme Specificity and Thermostability
ChemBioChem ( IF 3.2 ) Pub Date : 2018-04-14 , DOI: 10.1002/cbic.201700665 Jianfeng Wang 1, 2 , Zhongmei Liu 1 , Zhemin Zhou 1
ChemBioChem ( IF 3.2 ) Pub Date : 2018-04-14 , DOI: 10.1002/cbic.201700665 Jianfeng Wang 1, 2 , Zhongmei Liu 1 , Zhemin Zhou 1
Affiliation
|
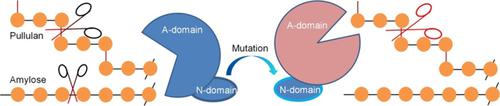
Enzyme engineering: The N‐terminal domain of the amylopullulanase PulWB42 is identified as carbohydrate‐binding module 68, in which residue 90 or 93 acts as a trigger to convert amylopullulanase into type I pullulanase, and the His5–Arg6–Thr7 segment or Gln87 impacts enzyme thermostability.

中文翻译:

Anoxybacillus sp。的支链淀粉酶的N末端结构域。WB42调节酶特异性和热稳定性
酶工程:淀粉葡聚糖酶PulWB42的N末端结构域被确定为碳水化合物结合模块68,其中残基90或93触发将淀粉葡聚糖酶转化为I型支链淀粉酶,并且His5-Arg6-Thr7节段或Gln87影响酶热稳定性。

更新日期:2018-04-14

中文翻译:

Anoxybacillus sp。的支链淀粉酶的N末端结构域。WB42调节酶特异性和热稳定性
酶工程:淀粉葡聚糖酶PulWB42的N末端结构域被确定为碳水化合物结合模块68,其中残基90或93触发将淀粉葡聚糖酶转化为I型支链淀粉酶,并且His5-Arg6-Thr7节段或Gln87影响酶热稳定性。




























 京公网安备 11010802027423号
京公网安备 11010802027423号