Catalysis Today ( IF 5.3 ) Pub Date : 2018-03-01 , DOI: 10.1016/j.cattod.2018.02.054 Michael Zeets , Daniel E. Resasco , Bin Wang
|
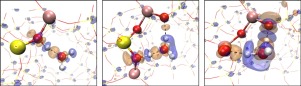
The adjustable acid sites in zeolites and its well-defined pore structure allows for a fine-tuning of the catalytic performance. The activity and selectivity of several reactions have been shown to be dependent on the location and distribution of the acid sites in the zeolite. However, the underlying mechanisms responsible for this dependence remain to be explored. Here, we report density functional theory calculations, through which we investigate the impact of proximity of Brønsted acid sites in the HZSM-5. We find that Brønsted sites with close spatial proximity can significantly strengthen the adsorption of water, which is used as a molecular probe for the local activity. We find that a water molecule can form H-bonds with two adjacent sites with increased adsorption energy. This increase is attributed to enhanced polarization of the water molecule through pronounced interfacial charge transfer. In some case, we observe that the proton becomes detached from the zeolite framework and forms a hydronium ion. We further show that hybrid functional calculations are essential for accurately describing the structural stability when two sites are located in close proximity of each other. Both, the enhanced polarization and proton delocalization may affect activity and selectivity for zeolite-catalyzed reactions.
中文翻译:

HZSM-5中相邻的布朗斯台德酸中心的化学活性和润湿性增强
沸石中可调节的酸位及其明确的孔结构可实现催化性能的微调。已经表明几种反应的活性和选择性取决于沸石中酸位的位置和分布。但是,导致这种依赖性的潜在机制仍有待探索。在这里,我们报告密度泛函理论计算,通过它我们研究了HZSM-5中布朗斯台德酸位点附近的影响。我们发现,空间接近的布朗斯台德站点可以显着增强水的吸附,水被用作局部活动的分子探针。我们发现,水分子可以与两个相邻的位点形成H键,并增加吸附能。这种增加归因于通过明显的界面电荷转移而使水分子的极化增强。在某些情况下,我们观察到质子从沸石骨架上脱离并形成水合氢离子。我们进一步表明,当两个位置彼此紧邻时,混合功能计算对于准确描述结构稳定性至关重要。增强的极化作用和质子离域作用都可能影响沸石催化反应的活性和选择性。我们进一步表明,当两个位置彼此紧邻时,混合功能计算对于准确描述结构稳定性至关重要。增强的极化作用和质子离域作用都可能影响沸石催化反应的活性和选择性。我们进一步表明,当两个位置彼此紧邻时,混合功能计算对于准确描述结构稳定性至关重要。增强的极化作用和质子离域作用都可能影响沸石催化反应的活性和选择性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号