当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Interconversion between KSc2F7:Yb/Er and K2NaScF6:Yb/Er nanocrystals: the role of chemistry†
Dalton Transactions ( IF 4 ) Pub Date : 2018-02-28 00:00:00 , DOI: 10.1039/c7dt04658h Yangbo Wang 1, 2, 3, 4, 5 , Bingxiao Yang 1, 2, 3, 4, 5 , Kun Chen 1, 2, 3, 4, 5 , Enlong Zhou 1, 2, 3, 4, 5 , Qinghua Zhang 6, 7, 8, 9, 10 , Lisha Yin 1, 2, 3, 4, 5 , Xiaoji Xie 1, 2, 3, 4, 5 , Lin Gu 6, 7, 8, 9, 10 , Ling Huang 1, 2, 3, 4, 5
Dalton Transactions ( IF 4 ) Pub Date : 2018-02-28 00:00:00 , DOI: 10.1039/c7dt04658h Yangbo Wang 1, 2, 3, 4, 5 , Bingxiao Yang 1, 2, 3, 4, 5 , Kun Chen 1, 2, 3, 4, 5 , Enlong Zhou 1, 2, 3, 4, 5 , Qinghua Zhang 6, 7, 8, 9, 10 , Lisha Yin 1, 2, 3, 4, 5 , Xiaoji Xie 1, 2, 3, 4, 5 , Lin Gu 6, 7, 8, 9, 10 , Ling Huang 1, 2, 3, 4, 5
Affiliation
|
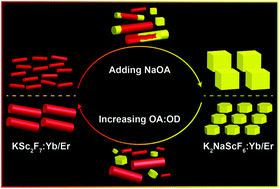
Scandium (Sc) sits at a unique position in the periodic table, i.e., the junction of the top of the rare earth column and the beginning of the transition metal row. Studies have shown that Sc-based nanomaterials are very sensitive to the surrounding chemical environment. A simple adjustment of the chemical reaction conditions such as temperature, surfactant molecules, and solvents (e.g., oleic acid (OA) or 1-octadecene (OD)) can easily lead to different products in terms of chemical composition and phase structure. Herein, under purposely adjusted reaction conditions, we have investigated the interconversion process between two representative Sc-based nanomaterials, that is, nanocrystals of orthorhombic KSc2F7:Yb/Er and cubic K2NaScF6:Yb/Er, both of which have characteristic red upconversion luminescence and high similarity in chemical composition and phase structure. Experimental results have indicated that conversion from KSc2F7:Yb/Er to K2NaScF6:Yb/Er may start from the edge of the nanocrystal where K+ in KSc2F7:Yb/Er was gradually substituted by the post-introduced Na+ in the solution and finally KSc2F7:Yb/Er nanorods were broken and K2NaScF6:Yb/Er nanocubes were formed. On the other hand, a simple variation of the OA : OD ratio facilitates the dissolution of K2NaScF6:Yb/Er and subsequent crystallization of KSc2F7:Yb/Er during the opposite conversion process. Possible chemical reaction mechanisms were further developed to elucidate the interconversion details. Meanwhile, the variation of the upconversion luminescence such as emission intensity, red to green ratio, and lifetime is interpreted to monitor the conversion progress at corresponding stages, which is highly consistent with the scenario discussed above.
中文翻译:

KSc 2 F 7:Yb / Er和K 2 NaScF 6:Yb / Er纳米晶体之间的相互转化:化学作用†
dium(Sc)位于元素周期表中的唯一位置,即稀土柱顶部与过渡金属行的起点的交界处。研究表明,基于Sc的纳米材料对周围的化学环境非常敏感。简单调整化学反应条件,例如温度,表面活性剂分子和溶剂(例如,油酸(OA)或1-十八碳烯(OD)),就化学组成和相结构而言,很容易导致产生不同的产物。本文中,在有针对性地调整反应条件下,我们研究了两种代表性的Sc基纳米材料之间的相互转化过程,即正交晶体KSc 2 F 7:Yb / Er和立方K2 NaScF 6:Yb / Er,两者均具有特征性的红色上转换发光,并且在化学组成和相结构上具有高度相似性。实验结果表明,从KSc 2 F 7:Yb / Er到K 2 NaScF 6:Yb / Er的转化可能始于纳米晶体的边缘,其中KSc 2 F 7:Yb / Er中的K +逐渐被柱取代。溶液中引入Na +,最后破坏KSc 2 F 7:Yb / Er纳米棒,并破坏K 2 NaScF 6:形成Yb / Er纳米立方体。另一方面,在相反的转化过程中,OA:OD比的简单变化促进了K 2 NaScF 6:Yb / Er的溶解和随后的KSc 2 F 7:Yb / Er的结晶。进一步开发了可能的化学反应机理以阐明相互转化的细节。同时,上转换发光的变化(例如发射强度,红绿比和寿命)被解释为监视相应阶段的转换进度,这与上面讨论的情况高度一致。
更新日期:2018-02-28
中文翻译:

KSc 2 F 7:Yb / Er和K 2 NaScF 6:Yb / Er纳米晶体之间的相互转化:化学作用†
dium(Sc)位于元素周期表中的唯一位置,即稀土柱顶部与过渡金属行的起点的交界处。研究表明,基于Sc的纳米材料对周围的化学环境非常敏感。简单调整化学反应条件,例如温度,表面活性剂分子和溶剂(例如,油酸(OA)或1-十八碳烯(OD)),就化学组成和相结构而言,很容易导致产生不同的产物。本文中,在有针对性地调整反应条件下,我们研究了两种代表性的Sc基纳米材料之间的相互转化过程,即正交晶体KSc 2 F 7:Yb / Er和立方K2 NaScF 6:Yb / Er,两者均具有特征性的红色上转换发光,并且在化学组成和相结构上具有高度相似性。实验结果表明,从KSc 2 F 7:Yb / Er到K 2 NaScF 6:Yb / Er的转化可能始于纳米晶体的边缘,其中KSc 2 F 7:Yb / Er中的K +逐渐被柱取代。溶液中引入Na +,最后破坏KSc 2 F 7:Yb / Er纳米棒,并破坏K 2 NaScF 6:形成Yb / Er纳米立方体。另一方面,在相反的转化过程中,OA:OD比的简单变化促进了K 2 NaScF 6:Yb / Er的溶解和随后的KSc 2 F 7:Yb / Er的结晶。进一步开发了可能的化学反应机理以阐明相互转化的细节。同时,上转换发光的变化(例如发射强度,红绿比和寿命)被解释为监视相应阶段的转换进度,这与上面讨论的情况高度一致。



























 京公网安备 11010802027423号
京公网安备 11010802027423号