当前位置:
X-MOL 学术
›
Eur. Polym. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Lead (II) removal by poly(N,N-dimethylacrylamide-co-2-hydroxyethyl methacrylate)
European Polymer Journal ( IF 6 ) Pub Date : 2018-04-01 , DOI: 10.1016/j.eurpolymj.2018.02.032 A.L. Ramos-Jacques , J.A. Lujan-Montelongo , C. Silva-Cuevas , M. Cortez-Valadez , M. Estevez , A.R. Hernandez-Martínez
European Polymer Journal ( IF 6 ) Pub Date : 2018-04-01 , DOI: 10.1016/j.eurpolymj.2018.02.032 A.L. Ramos-Jacques , J.A. Lujan-Montelongo , C. Silva-Cuevas , M. Cortez-Valadez , M. Estevez , A.R. Hernandez-Martínez
|
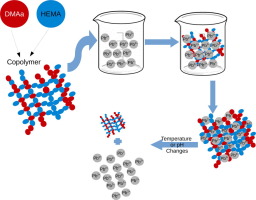
Abstract Traces of certain heavy metals are crucial for the metabolism of living beings, but poisoning occurs when their concentration increases. On the other hand, heavy metals such as lead, mercury, and cadmium lack functionality in higher organisms, resulting in considerable toxicity. Their long-term persistence is an environmental issue, being adsorption an alternative for the removal of metal pollutants, for example in wastewater. Adsorption has many advantages, such as high efficiency, simple design, low toxicity and reusable materials. Among the adsorbents, hydrogels feature improved resistance and high levels of swelling, comparable in costs to other common materials. In this work, we synthesized a copolymer of N,N-dimethylacrylamide (DMAa) and 2-hydroxyethyl methacrylate (HEMA) and evaluated its lead (II) adsorption capacity. The copolymer poly(HEMA-co-DMAa) was synthesized by a free-radical type bulk copolymerization of equal volumes of each monomer. The copolymer was characterized by 1H and 13C NMR, and Raman spectroscopies, and by the temperature and pH dependant-swelling effect of the hydrogel. Quantification of the removed Pb2+ from probe solutions was performed by a UV spectrophotometric method as proposed by Krzek et al. Results suggest that at equilibrium, the amount of sorbed Pb(II) increased as the probe solution also increased (gradually from 10 to 200 ppm). Raman and 13C spectroscopies confirmed effective copolymerization between HEMA and DMAa. The p(HEMA-co-DMAa) copolymer featured a swelling capacity up to 3.6 times their weight of water and exhibited a LCST in the range of 308–340 K with its geometric center around 313 K. In conclusion, a thermo-and-pH-responsive copolymer was obtained from the HEMA and DMAa monomers with LCST at 313 K and pH critical point at pH = 8, featuring efficient Pb(II) adsorption capabilities. The synthesized p(HEMA-co-DMAa) is promising for applications in heavy metals adsorption in aqueous systems with a broad range of pH and temperature, featuring resistance on adsorption and desorption cycles without mechanical degradation.
中文翻译:

聚(N,N-二甲基丙烯酰胺-co-2-羟乙基甲基丙烯酸酯)去除铅(II)
摘要 某些重金属的微量对生物的新陈代谢至关重要,但当其浓度增加时就会发生中毒。另一方面,铅、汞、镉等重金属在高等生物中缺乏功能,导致相当大的毒性。它们的长期持久性是一个环境问题,吸附是去除金属污染物(例如废水中的金属污染物)的替代方法。吸附具有效率高、设计简单、毒性低、材料可重复使用等诸多优点。在吸附剂中,水凝胶具有更高的阻力和高溶胀度,其成本可与其他常见材料相媲美。在这项工作中,我们合成了 N,N-二甲基丙烯酰胺 (DMAa) 和甲基丙烯酸 2-羟乙酯 (HEMA) 的共聚物,并评估了其铅 (II) 吸附能力。共聚物聚(HEMA-co-DMAa)是通过等体积的每种单体的自由基型本体共聚反应合成的。该共聚物的特征在于 1H 和 13C NMR、拉曼光谱以及水凝胶的温度和 pH 依赖性溶胀效应。从探针溶液中去除的 Pb2+ 的定量是通过 Krzek 等人提出的紫外分光光度法进行的。结果表明,在平衡状态下,吸附的 Pb(II) 量随着探针溶液的增加而增加(从 10 ppm 逐渐增加到 200 ppm)。拉曼和 13C 光谱证实了 HEMA 和 DMAa 之间的有效共聚。p(HEMA-co-DMAa) 共聚物的溶胀能力高达其水重量的 3.6 倍,其 LCST 范围为 308-340 K,其几何中心约为 313 K。 总之,从 HEMA 和 DMAa 单体获得了一种热响应和 pH 响应共聚物,LCST 为 313 K,pH 临界点为 pH = 8,具有高效的 Pb(II) 吸附能力。合成的 p(HEMA-co-DMAa) 有望在广泛的 pH 值和温度范围内应用于水性体系中的重金属吸附,具有抗吸附和解吸循环而无机械降解的特点。
更新日期:2018-04-01
中文翻译:

聚(N,N-二甲基丙烯酰胺-co-2-羟乙基甲基丙烯酸酯)去除铅(II)
摘要 某些重金属的微量对生物的新陈代谢至关重要,但当其浓度增加时就会发生中毒。另一方面,铅、汞、镉等重金属在高等生物中缺乏功能,导致相当大的毒性。它们的长期持久性是一个环境问题,吸附是去除金属污染物(例如废水中的金属污染物)的替代方法。吸附具有效率高、设计简单、毒性低、材料可重复使用等诸多优点。在吸附剂中,水凝胶具有更高的阻力和高溶胀度,其成本可与其他常见材料相媲美。在这项工作中,我们合成了 N,N-二甲基丙烯酰胺 (DMAa) 和甲基丙烯酸 2-羟乙酯 (HEMA) 的共聚物,并评估了其铅 (II) 吸附能力。共聚物聚(HEMA-co-DMAa)是通过等体积的每种单体的自由基型本体共聚反应合成的。该共聚物的特征在于 1H 和 13C NMR、拉曼光谱以及水凝胶的温度和 pH 依赖性溶胀效应。从探针溶液中去除的 Pb2+ 的定量是通过 Krzek 等人提出的紫外分光光度法进行的。结果表明,在平衡状态下,吸附的 Pb(II) 量随着探针溶液的增加而增加(从 10 ppm 逐渐增加到 200 ppm)。拉曼和 13C 光谱证实了 HEMA 和 DMAa 之间的有效共聚。p(HEMA-co-DMAa) 共聚物的溶胀能力高达其水重量的 3.6 倍,其 LCST 范围为 308-340 K,其几何中心约为 313 K。 总之,从 HEMA 和 DMAa 单体获得了一种热响应和 pH 响应共聚物,LCST 为 313 K,pH 临界点为 pH = 8,具有高效的 Pb(II) 吸附能力。合成的 p(HEMA-co-DMAa) 有望在广泛的 pH 值和温度范围内应用于水性体系中的重金属吸附,具有抗吸附和解吸循环而无机械降解的特点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号