Journal of Controlled Release ( IF 10.8 ) Pub Date : 2018-02-28 , DOI: 10.1016/j.jconrel.2018.02.039 Santhosh Kalash Rajendrakumar , Kondareddy Cherukula , Hyeong Ju Park , Saji Uthaman , Yong Yeon Jeong , Byeong-Il Lee , In-Kyu Park
|
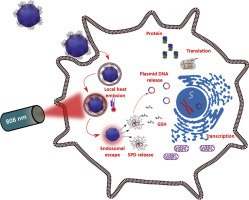
Stimuli-responsive polymeric nanoparticles are useful for overcoming challenges such as transfection efficiency and the specific and safe delivery of genes to cancer cells. Transfection outcomes can be improved through spatially and temporally controlled gene release. We formulated a nanoassembly comprising a disulfide-crosslinked polyethylenimine (ssPEI) conjugated with a tumor-specific cell-penetrating peptide (DS 4-3) (SPD) polyplex and bovine serum albumin (BSA)-loaded IR780 (BI) nanoparticle, thereby forming a dual-stimulus-triggered, tumor-penetrating and gene-carrying nanoassembly (BI-SPD) via electrostatic complexing. BI-SPD nanoassembly were composed of highly stable nanosized complexes with an average size of 457 ± 27.5 nm, exhibiting an up to two-fold enhanced transfection efficiency with no sign of potential cytotoxicity in breast cancer cells. Moreover, upon laser irradiation, a four-fold increase in transfection efficiency was achieved due to the rapid endosomal escape of polyplexes triggered by the local heat induced by the BI-SPD nanoassembly. Additionally, the high redox environment in tumor cells facilitated the disassembly of the SPD polyplex for efficient plasmid release in the cytosol. The BI-SPD nanoassembly also exhibited high penetration and enhanced photothermally triggered gene expression in the 4T1 spheroid model. This BI-SPD nanoassembly has the potential to enhance the expression of therapeutic genes in tumor models without causing significant toxicity to surrounding healthy tissues, since it has shown higher tumor targeting and accumulation in the 4T1 tumor in mice model.
中文翻译:

双重刺激响应的白蛋白-多聚体纳米组装转移性乳腺癌的空间控制基因释放。
刺激响应性聚合物纳米颗粒可用于克服挑战,例如转染效率以及基因向癌细胞的特异性和安全传递。转染的结果可以通过空间和时间控制的基因释放来改善。我们配制了一种纳米组件,该组件包含与肿瘤特异性细胞穿透肽(DS 4-3)(SPD)多态复合物和牛血清白蛋白(BSA)负载的IR780(BI)纳米粒子缀合的二硫键交联的聚乙烯亚胺(ssPEI)。通过静电复合的双重刺激触发,肿瘤穿透和携带基因的纳米组装(BI-SPD)。BI-SPD纳米组件由高度稳定的纳米复合物组成,平均尺寸为457±27.5 nm,表现出高达两倍的转染效率,在乳腺癌细胞中没有潜在的细胞毒性迹象。此外,在激光照射下,由于BI-SPD纳米组装体诱导的局部热量触发了复合体的快速内体逸出,因此转染效率提高了四倍。另外,肿瘤细胞中的高氧化还原环境促进了SPD多聚体的分解,以使质粒有效地释放到细胞质中。BI-SPD纳米装配体在4T1球体模型中还表现出高渗透性和增强的光热触发基因表达。这种BI-SPD纳米装配体具有增强治疗性基因在肿瘤模型中表达的潜力,而不会对周围的健康组织造成明显的毒性,


























 京公网安备 11010802027423号
京公网安备 11010802027423号