当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
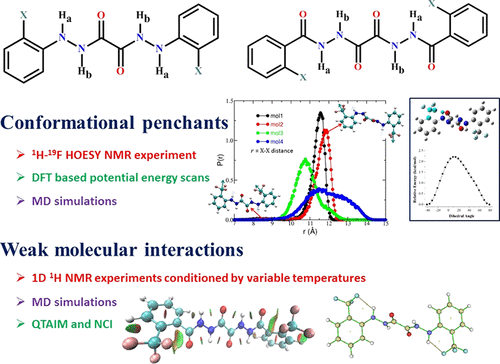
Intramolecular Hydrogen Bonding Appetency for Conformational Penchants in Oxalohydrazide Fluoro Derivatives: NMR, MD, QTAIM, and NCI Studies
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-02-28 00:00:00 , DOI: 10.1021/acs.jpca.8b00913 A. Lakshmipriya 1, 2 , Madhusudan Chaudhary 1 , Santosh Mogurampelly 3 , Michael L. Klein 3 , N. Suryaprakash 1, 2
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-02-28 00:00:00 , DOI: 10.1021/acs.jpca.8b00913 A. Lakshmipriya 1, 2 , Madhusudan Chaudhary 1 , Santosh Mogurampelly 3 , Michael L. Klein 3 , N. Suryaprakash 1, 2
Affiliation
|
The conformational stability of synthesized diphenyloxalohydrazide and dibenzoyloxalohydrazide fluoro derivatives has been investigated by extensive NMR studies that are ascertained by various levels of theoretical calculations. Two-dimensional 1H–19F HOESY NMR experiments revealed the close spatial proximity between two NMR-active nuclei, confirming the hydrogen bond (HB)-mediated interaction between them, further aiding in establishing the probable stable conformations of these molecules. The relaxed potential energy scan disclosed the energy-minimized most stable structure among the several possible multiple conformations, which is in concurrence with NMR interpretations. Atomistic molecular dynamics simulations have been employed to unequivocally establish the conformational stability and the nature of HB formation at varied temperatures. With the possibility of occurrence of a number of probable conformations, the percentage of occurrences of different types of HBs in them was determined by MD simulations. Their population analysis was carried out using a Boltzmann distribution, in addition to deriving their Gibbs free energies. The molecular interactions governing the stable conformations have not only been ascertained by experimental NMR interpretations but also corroborated by other theoretical computations, viz., quantum theory of atoms in molecules (QTAIM) and noncovalent interaction (NCI).
中文翻译:

草酰肼氟衍生物中构象结合剂的分子内氢键结合能力:NMR,MD,QTAIM和NCI研究
合成的二苯基草酰肼和二苯甲酰草酰肼氟衍生物的构象稳定性已通过广泛的NMR研究进行了研究,该研究由各种水平的理论计算确定。二维1 H– 19F HOESY NMR实验揭示了两个NMR活性核之间的紧密空间邻近性,证实了它们之间氢键(HB)介导的相互作用,进一步有助于建立这些分子的可能稳定构象。宽松的势能扫描揭示了几种可能的多重构象中能量最小的最稳定结构,这与NMR解释是一致的。原子分子动力学模拟已被用来明确确定构象稳定性和在不同温度下HB形成的性质。由于可能会出现许多可能的构象,因此通过MD模拟确定了其中不同类型HBs出现的百分比。他们的人口分析是使用Boltzmann分布进行的,除了获得吉布斯自由能之外 支配稳定构象的分子相互作用不仅已通过实验NMR的解释得以确定,而且已通过其他理论计算得到证实,即分子中原子的量子理论(QTAIM)和非共价相互作用(NCI)。
更新日期:2018-02-28
中文翻译:

草酰肼氟衍生物中构象结合剂的分子内氢键结合能力:NMR,MD,QTAIM和NCI研究
合成的二苯基草酰肼和二苯甲酰草酰肼氟衍生物的构象稳定性已通过广泛的NMR研究进行了研究,该研究由各种水平的理论计算确定。二维1 H– 19F HOESY NMR实验揭示了两个NMR活性核之间的紧密空间邻近性,证实了它们之间氢键(HB)介导的相互作用,进一步有助于建立这些分子的可能稳定构象。宽松的势能扫描揭示了几种可能的多重构象中能量最小的最稳定结构,这与NMR解释是一致的。原子分子动力学模拟已被用来明确确定构象稳定性和在不同温度下HB形成的性质。由于可能会出现许多可能的构象,因此通过MD模拟确定了其中不同类型HBs出现的百分比。他们的人口分析是使用Boltzmann分布进行的,除了获得吉布斯自由能之外 支配稳定构象的分子相互作用不仅已通过实验NMR的解释得以确定,而且已通过其他理论计算得到证实,即分子中原子的量子理论(QTAIM)和非共价相互作用(NCI)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号