Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2018-02-27 , DOI: 10.1016/j.bmc.2018.02.048 Zafer Sahin , Merve Ertas , Barkın Berk , Sevde Nur Biltekin , Leyla Yurttas , Seref Demirayak
|
Steroidal and non-steroidal aromatase inhibitors target the suppression of estrogen biosynthesis in the treatment of breast cancer. Researchers have increasingly focused on developing non-steroidal derivatives for their potential clinical use avoiding steroidal side-effects.
Non-steroidal derivatives generally have planar aromatic structures attached to the azole ring system. One part of this ring system comprises functional groups that inhibit aromatization through the coordination of the haem group of the aromatase enzyme. Replacement of the triazole ring system and development of aromatic/cyclic structures of the side chain can increase selectivity over aromatase enzyme inhibition.
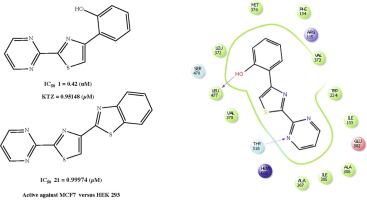
In this study, 4-(aryl/heteroaryl)-2-(pyrimidin-2-yl)thiazole derivatives were synthesized and physical analyses and structural determination studies were performed. The IC50 values were determined by a fluorescence-based aromatase inhibition assay and compound 1 (4-(2-hydroxyphenyl)-2-(pyrimidine-2-yl)thiazole) were found potent inhibitor of enzyme (IC50:0.42 nM). Then, their antiproliferative activity over MCF-7 and HEK-293 cell lines was evaluated using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Compounds 1, 7, 8, 13, 15, 18, 21 were active against MCF-7 breast cancer cells. Lastly, a series of docking experiments were undertaken to analyze the crystal structure of human placental aromatase and identify the possible interactions between the most active structure and the active site.
中文翻译:

非甾族芳香酶抑制剂的研究;4-(芳基/杂芳基)-2-(嘧啶-2-基)噻唑衍生物
甾体和非甾体芳香酶抑制剂的目标是在乳腺癌的治疗中抑制雌激素的生物合成。研究人员越来越多地致力于开发非甾体衍生物以用于其潜在的临床用途,从而避免甾体副作用。
非甾族衍生物通常具有连接至唑环系统的平面芳族结构。该环系统的一部分包含通过芳香化酶的血红素基团的配位抑制芳香化的官能团。三唑环系统的取代和侧链芳族/环状结构的发展可以增加对芳香酶抑制的选择性。
在这项研究中,合成了4-(芳基/杂芳基)-2-(嘧啶-2-基)噻唑衍生物,并进行了物理分析和结构确定研究。通过基于荧光的芳香化酶抑制测定法确定IC 50值,并且发现化合物1(4-(2-羟苯基)-2-(嘧啶-2-基)噻唑)是有效的酶抑制剂(IC 50:0.42 nM) 。然后,使用3-(4,5-二甲基噻唑-2-基)-2,5-二苯基四唑溴化物(MTT)分析评估了它们对MCF-7和HEK-293细胞系的抗增殖活性。化合物1,7,8,13,15,18,21对MCF-7乳腺癌细胞具有活性。最后,进行了一系列的对接实验,以分析人胎盘芳香酶的晶体结构,并确定最活跃的结构与活跃位点之间可能存在的相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号