Applied Catalysis A: General ( IF 5.5 ) Pub Date : 2018-02-27 , DOI: 10.1016/j.apcata.2018.02.031 E. Inkeri Kauppi , Ella H. Rönkkönen , Jouko Lahtinen , A. Outi Krause
|
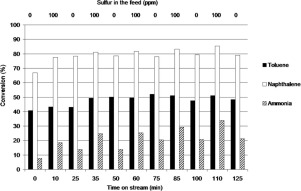
Use of the syngas produced by biomass gasification is rendered unviable because of impurity compounds, whose efficient removal is key to the feasibility of gasification concepts. Tars present in the biomass gasification gas can be efficiently oxidized by ZrO2 catalysts. H2S has been shown to increase the oxidation activity on ZrO2 and most notably on La2O3-ZrO2. Gasification gas clean-up experiments were performed at 600–800 °C to study the beneficial effect of H2S on naphthalene and toluene conversions. During time-on-stream with repeated doses of H2S on ZrO2, cumulative and strong H2S adsorption was observed as the conversion of toluene and naphthalene increased during the first H2S doses and stabilized towards the end. Therefore, a limited capacity of the catalyst to adsorb H2S was suggested, which is the origin of sulfur tolerance. Activity enhancement with H2S occurs most notably on La2O3-ZrO2 and towards naphthalene oxidation. XPS characterization of the used catalysts showed sulfur retention on the La2O3-ZrO2 but not on pure ZrO2, indicating that La2O3-ZrO2 holds more sulfur on its surface or binds it stronger. The surface properties of La2O3-ZrO2 were thus characterized with in situ DRIFTS using CO2 and CH3OH as probe molecules. The surface of La2O3-ZrO2 was suggested to have more low coordination sites than ZrO2, where sulfur is suggested to be bound and alter the reactivity of the surface oxygen.
中文翻译:

H 2 S对生物质气化气净化中ZrO 2和La 2 O 3 -ZrO 2催化剂性能的促进作用
由于杂质化合物的存在,使用生物质气化生产的合成气变得不可行,杂质化合物的有效去除是气化概念可行性的关键。ZrO 2催化剂可以有效地氧化生物质气化气中存在的焦油。已经表明,H 2 S增加对ZrO 2的氧化活性,最明显的是对La 2 O 3 -ZrO 2的氧化活性。在600–800°C下进行了气化气净化实验,以研究H 2 S对萘和甲苯转化的有利影响。在重复使用Hr 2 S的ZrO 2上的运行时间中,累积的H 2和强H 2在第一批H 2 S剂量期间,随着甲苯和萘的转化率增加并最终趋于稳定,观察到了S吸附。因此,提出了催化剂吸附H 2 S的能力有限,这是耐硫性的根源。H 2 S的活性增强最明显地发生在La 2 O 3 -ZrO 2上并向萘氧化。用过的催化剂的XPS表征表明,硫保留在La 2 O 3 -ZrO 2上,但不保留在纯ZrO 2上,表明La 2 O 3 -ZrO 2在其表面上保留更多的硫或使其更牢固地结合。因此,使用CO 2和CH 3 OH作为探针分子,通过原位DRIFTS表征了La 2 O 3 -ZrO 2的表面性质。La 2 O 3 -ZrO 2的表面被认为比ZrO 2具有更低的配位点,ZrO 2被认为会结合硫并改变表面氧的反应性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号