Applied Catalysis A: General ( IF 5.5 ) Pub Date : 2018-02-27 , DOI: 10.1016/j.apcata.2018.02.033 Magdalena Markiton , Anna Szelwicka , Sławomir Boncel , Sebastian Jurczyk , Anna Chrobok
|
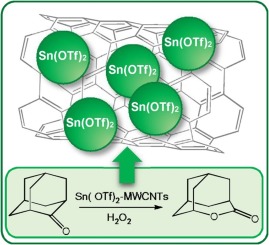
The Baeyer-Villiger oxidation of a model cyclic ketone, 2-adamantanone, using H2O2 as the oxidizing agent, was systematically studied using a range of metal triflates in toluene. The extremely high activity of Sn(OTf)2 in promoting the oxidation, allowing full conversion of the ketone and lactone formation in a very short time (20 min, molar ratio Sn(OTf)2:ketone:30 wt% aq. H2O2 = 0.1:1.0:2.0 at 70 °C) was reported for the first time. Next, Sn(OTf)2 was used as an active phase and immobilized on various (nano)carbon materials. The application of the most active catalyst using multi-walled carbon nanotubes (MWCNTs) (length 1.5 μm, outer diameter 9.5 nm) as a carrier allowed to reduce the amount of the active phase (Sn(OTf)2) to 0.26 mol% per ketone (full conversion of ketone to lactone was achieved after 20 min at 90 °C for molar ratio catalyst:ketone:30 wt% aq. H2O2: 0.0026:1.00:2.00, or full conversion of ketone to lactone was achieved after 1 h at 70 °C for molar ratio catalyst:ketone:60 wt% aq. H2O2: 0.0026:1.00:1.50). The superactivity was related to spherical nanosize Sn(OTf)2 particles (ca. 2 nm) dispersed along the outer nanotube walls. The participation of both Lewis and Brønsted acids sites in the conversion of the ketone was postulated. A simple test with the generation of a retinyl cation shown that Sn(OTf)2 underwent partial hydrolysis to triflic acid.
中文翻译:

用于Baeyer-Villiger氧化的超活性三氟甲磺酸锡(II)/碳纳米管催化剂
使用一系列在甲苯中的金属三氟甲磺酸,系统地研究了使用H 2 O 2作为氧化剂对模型环状酮2-金刚烷酮的Baeyer-Villiger氧化。Sn(OTf)2具有极高的促进氧化的活性,可在非常短的时间内(20分钟,Sn(OTf)2:酮:30 wt%H 2水溶液)完全转化酮和内酯。 首次报告在70°C时O 2 = 0.1:1.0:2.0。接下来,Sn(OTf)2将其用作活性相并固定在各种(纳米)碳材料上。使用多壁碳纳米管(MWCNT)(长度1.5μm,外径9.5 nm)作为载体的活性最高的催化剂可以将活性相(Sn(OTf)2)的量减少至每摩尔0.26 mol%酮(在90°C下20分钟后,对于催化剂:酮:30 wt%H 2 O 2水溶液:0.0026:1.00:2.00的摩尔比,酮完全转化为内酯,或者在反应后将酮完全转化为内酯对于70:1摩尔比的催化剂:酮:60 wt%H 2 O 2水溶液:0.0026:1.00:1.50)。超活性与球形纳米Sn(OTf)2有关颗粒(约2 nm)沿纳米管外壁分散。假设路易斯酸和布朗斯台德酸位点都参与了酮的转化。一个简单的生成视黄酸阳离子的测试表明,Sn(OTf)2经历了部分水解为三氟甲磺酸。



























 京公网安备 11010802027423号
京公网安备 11010802027423号