当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
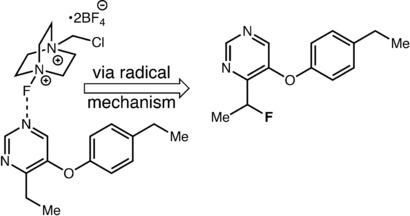
Benzylic Fluorination of Aza‐Heterocycles Induced by Single‐Electron Transfer to Selectfluor
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-03-22 , DOI: 10.1002/anie.201801280 Kelley E Danahy 1 , Julian C Cooper 1 , Jeffrey F Van Humbeck 2
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-03-22 , DOI: 10.1002/anie.201801280 Kelley E Danahy 1 , Julian C Cooper 1 , Jeffrey F Van Humbeck 2
Affiliation
|
A selective and mild method for the benzylic fluorination of aromatic azaheterocycles with Selectfluor is described. These reactions take place by a previously unreported mechanism, in which electron transfer from the heterocyclic substrate to the electrophilic fluorinating agent Selectfluor eventually yields a benzylic radical, thus leading to the desired C−F bond formation. This mechanism enables high intra‐ and intermolecular selectivity for aza‐heterocycles over other benzylic components with similar C−H bond‐dissociation energies.
中文翻译:

单电子转移至 Selectfluor 诱导氮杂杂环的苄基氟化
描述了一种使用 Selectfluor 对芳香氮杂杂环进行苄基氟化的选择性温和方法。这些反应通过以前未报道的机制发生,其中电子从杂环底物转移到亲电子氟化剂 Selectfluor 最终产生苄基自由基,从而导致所需的 CF 键形成。这种机制使氮杂杂环比具有相似 C-H 键解离能的其他苄基组分具有更高的分子内和分子间选择性。
更新日期:2018-03-22
中文翻译:

单电子转移至 Selectfluor 诱导氮杂杂环的苄基氟化
描述了一种使用 Selectfluor 对芳香氮杂杂环进行苄基氟化的选择性温和方法。这些反应通过以前未报道的机制发生,其中电子从杂环底物转移到亲电子氟化剂 Selectfluor 最终产生苄基自由基,从而导致所需的 CF 键形成。这种机制使氮杂杂环比具有相似 C-H 键解离能的其他苄基组分具有更高的分子内和分子间选择性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号