当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Understanding the Catalase-Like Activity of a Bioinspired Manganese(II) Complex with a Pentadentate NSNSN Ligand Framework. A Computational Insight into the Mechanism
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-02-27 00:00:00 , DOI: 10.1021/acscatal.7b03559 Maria Letizia Merlini 1 , George J. P. Britovsek 2 , Marcel Swart 3, 4 , Paola Belanzoni 5, 6, 7
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-02-27 00:00:00 , DOI: 10.1021/acscatal.7b03559 Maria Letizia Merlini 1 , George J. P. Britovsek 2 , Marcel Swart 3, 4 , Paola Belanzoni 5, 6, 7
Affiliation
|
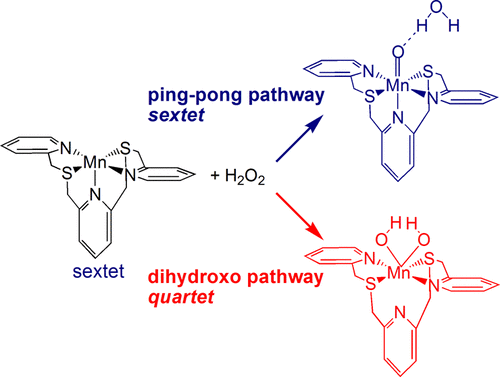
The mechanism of H2O2 dismutation catalyzed by the recently reported 2,6-bis[((2-pyridylmethyl)thio)methyl]pyridine-Mn(II) complex ([MnS2Py3(OTf)2]) has been investigated by density functional theory using the S12g functional. The complex has been analyzed in terms of its coordination properties and the reaction of [MnS2Py3]2+ in a distorted square pyramidal coordination geometry with two hydrogen peroxide molecules has been investigated in our calculations. The sextet, quartet, and doublet potential energy profiles of the catalytic reaction have been explored. In the first dismutation process, the rate-determining step (RDS) is found to be the asymmetric O–O bond cleavage, which occurs on the sextet potential energy profile. A subsequent spin crossover from sextet to quartet, associated with a coordination rearrangement around the metal, can take place to generate a stable Mn(IV) dihydroxo intermediate. This could disfavor the ping-pong mechanism commonly considered to describe the H2O2 dismutation reaction, where the binding of the first H2O2 substrate leads to the release of one H2O product and the conversion of the catalyst into a Mn(IV) oxo complex. The formation of this stable intermediate, featuring a peculiar trigonal prismatic coordination geometry, paves the way for an alternative reaction pathway for the second dismutation process, termed the dihydroxo mechanism, where two water molecules and dioxygen are easily and simultaneously formed. The competing channels have different spin states: the sextet reaction pathway corresponds to the ping-pong mechanism, whereas the quartet reaction follows preferably the dihydroxo mechanism. The doublet reaction path is energetically disfavored for both channels. For the ping-pong mechanism, the RDS in the second dismutation process is represented by the second hydrogen-abstraction from H2O2, with a calculated energy barrier very close to that of the RDS in the first dismutation reaction. Explicit solvent molecules, counterions, and trace amounts of water are found to further support the preference for the asymmetric O–O bond breaking by favoring the end-on coordination mode of the first H2O2 to the catalyst.
中文翻译:

了解具有五齿NSNSN配体框架的生物启发型锰(II)配合物的过氧化氢酶样活性。该机制的计算洞察力
最近报道的2,6-双[((2-吡啶基甲基)硫代)甲基]吡啶-Mn(II)配合物([MnS 2 Py 3(OTf)2 ])催化H 2 O 2歧化的机理是通过使用S12g泛函的密度泛函理论进行了研究。已对该配合物的配位性质和[MnS 2 Py 3 ] 2+的反应进行了分析。在我们的计算中,已经研究了具有两个过氧化氢分子的扭曲方锥体配位几何结构。已经研究了催化反应的六重态,四重态和双重态势能图。在第一个歧化过程中,速率确定步骤(RDS)被发现为不对称的O-O键断裂,发生在六重位势能曲线上。随后发生的自六方体到四方体的自旋交叉,与金属周围的配位重排有关,可生成稳定的Mn(IV)二羟基中间体。这可能不利于通常被认为描述H 2 O 2歧化反应的乒乓机制,其中第一H 2 O 2的结合底物导致一种H 2 O产物的释放以及催化剂向Mn(IV)羰基配合物的转化。这种稳定的中间体的形成,具有独特的三角棱柱形配位几何结构,为第二种歧化过程的另一种反应途径(称为二氢氧基机理)铺平了道路,在该途径中,两个水分子和双氧容易同时形成。竞争通道具有不同的自旋状态:六重奏反应途径对应于乒乓机制,而四重奏反应优选遵循二羟基机制。对于两个通道,双峰反应路径在能量上都是不利的。对于乒乓机制,第二个歧化过程中的RDS表示为第二次从H 2中吸氢O 2,其计算的能垒非常接近第一次歧化反应中的RDS。发现明确的溶剂分子,抗衡离子和痕量的水通过促进第一个H 2 O 2的端基配位模式促进了催化剂,从而进一步支持了不对称O-O键的断裂。
更新日期:2018-02-27
中文翻译:

了解具有五齿NSNSN配体框架的生物启发型锰(II)配合物的过氧化氢酶样活性。该机制的计算洞察力
最近报道的2,6-双[((2-吡啶基甲基)硫代)甲基]吡啶-Mn(II)配合物([MnS 2 Py 3(OTf)2 ])催化H 2 O 2歧化的机理是通过使用S12g泛函的密度泛函理论进行了研究。已对该配合物的配位性质和[MnS 2 Py 3 ] 2+的反应进行了分析。在我们的计算中,已经研究了具有两个过氧化氢分子的扭曲方锥体配位几何结构。已经研究了催化反应的六重态,四重态和双重态势能图。在第一个歧化过程中,速率确定步骤(RDS)被发现为不对称的O-O键断裂,发生在六重位势能曲线上。随后发生的自六方体到四方体的自旋交叉,与金属周围的配位重排有关,可生成稳定的Mn(IV)二羟基中间体。这可能不利于通常被认为描述H 2 O 2歧化反应的乒乓机制,其中第一H 2 O 2的结合底物导致一种H 2 O产物的释放以及催化剂向Mn(IV)羰基配合物的转化。这种稳定的中间体的形成,具有独特的三角棱柱形配位几何结构,为第二种歧化过程的另一种反应途径(称为二氢氧基机理)铺平了道路,在该途径中,两个水分子和双氧容易同时形成。竞争通道具有不同的自旋状态:六重奏反应途径对应于乒乓机制,而四重奏反应优选遵循二羟基机制。对于两个通道,双峰反应路径在能量上都是不利的。对于乒乓机制,第二个歧化过程中的RDS表示为第二次从H 2中吸氢O 2,其计算的能垒非常接近第一次歧化反应中的RDS。发现明确的溶剂分子,抗衡离子和痕量的水通过促进第一个H 2 O 2的端基配位模式促进了催化剂,从而进一步支持了不对称O-O键的断裂。

























 京公网安备 11010802027423号
京公网安备 11010802027423号