当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis, Application and Kinetic Studies of Chiral Phosphite‐Oxazoline Palladium Complexes as Active and Selective Catalysts in Intermolecular Heck Reactions
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-03-14 , DOI: 10.1002/adsc.201701603 Zahra Mazloomi 1 , Marc Magre 1 , Efrem Del Valle 1 , Miquel A. Pericàs 2, 3 , Oscar Pàmies 1 , Piet W. N. M. van Leeuwen 4 , Montserrat Diéguez 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-03-14 , DOI: 10.1002/adsc.201701603 Zahra Mazloomi 1 , Marc Magre 1 , Efrem Del Valle 1 , Miquel A. Pericàs 2, 3 , Oscar Pàmies 1 , Piet W. N. M. van Leeuwen 4 , Montserrat Diéguez 1
Affiliation
|
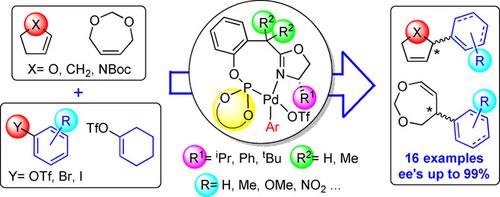
This study identifies new phosphite‐oxazoline ligands that have been successfully applied in the palladium‐catalyzed intermolecular asymmetric Heck reaction. The design of the new phosphite‐oxazoline ligands derives from a previous successful generation of phosphine‐oxazoline ligands, by replacing the phosphine group with several π‐acceptor biaryl phosphite moieties. With these simple modifications, the new phosphite‐based ligands, unlike previous phosphine‐oxazoline, not only present a modular design with numerous potential phosphite groups available, but they are also air‐stable solids, which can be made in the same number of synthetic steps as the phosphine analogues. The substitution of the phosphine by a biaryl phosphite group extended the range of substrates and triflates sources that can be coupled with regio‐, enantioselectivities and activities comparable to the few best ones reported. In addition, the ligands that provided the best selectivities contained an isopropyl oxazoline moiety instead of the tert‐butyl group found in the related phosphine‐oxazoline ligands, which is made from a much more expensive precursor. In this paper we have also carried out kinetic studies and a Hammett plot analysis to determine the rate determining step of this system in the regime of interest. We suggest a likely explanation for the fast Heck reaction of the phosphite‐oxazoline catalysts.
中文翻译:

手性亚磷酸-恶唑啉钯配合物作为分子间Heck反应的活性和选择性催化剂的合成,应用及动力学研究
这项研究确定了已成功用于钯催化的分子间不对称Heck反应的新型亚磷酸酯-恶唑啉配体。新的亚磷酸酯-恶唑啉配体的设计源自上一代成功的膦-恶唑啉配体,其通过用多个π-受体双芳基亚磷酸酯部分取代膦基来实现。通过这些简单的修改,与以前的膦-恶唑啉不同,新的基于亚磷酸酯的配体不仅提供了具有众多潜在亚磷酸酯基团的模块化设计,而且还是空气稳定的固体,可以通过相同数量的合成方法制得步骤作为膦类似物。用亚磷酸二芳基酯取代膦扩大了底物和三氟甲磺酸酯源的范围,这些底物和三氟甲磺酸源可与区域,对映选择性和活性可与报道的少数最佳方法和活性相媲美。另外,提供最佳选择性的配体包含异丙基恶唑啉部分,而不是在相关的膦-恶唑啉配体中发现了叔丁基,该叔丁基由昂贵得多的前体制成。在本文中,我们还进行了动力学研究和Hammett图分析,以确定该系统在感兴趣状态下的速率确定步骤。对于亚磷酸盐-恶唑啉催化剂的快速Heck反应,我们提出了一个可能的解释。
更新日期:2018-03-14
中文翻译:

手性亚磷酸-恶唑啉钯配合物作为分子间Heck反应的活性和选择性催化剂的合成,应用及动力学研究
这项研究确定了已成功用于钯催化的分子间不对称Heck反应的新型亚磷酸酯-恶唑啉配体。新的亚磷酸酯-恶唑啉配体的设计源自上一代成功的膦-恶唑啉配体,其通过用多个π-受体双芳基亚磷酸酯部分取代膦基来实现。通过这些简单的修改,与以前的膦-恶唑啉不同,新的基于亚磷酸酯的配体不仅提供了具有众多潜在亚磷酸酯基团的模块化设计,而且还是空气稳定的固体,可以通过相同数量的合成方法制得步骤作为膦类似物。用亚磷酸二芳基酯取代膦扩大了底物和三氟甲磺酸酯源的范围,这些底物和三氟甲磺酸源可与区域,对映选择性和活性可与报道的少数最佳方法和活性相媲美。另外,提供最佳选择性的配体包含异丙基恶唑啉部分,而不是在相关的膦-恶唑啉配体中发现了叔丁基,该叔丁基由昂贵得多的前体制成。在本文中,我们还进行了动力学研究和Hammett图分析,以确定该系统在感兴趣状态下的速率确定步骤。对于亚磷酸盐-恶唑啉催化剂的快速Heck反应,我们提出了一个可能的解释。



























 京公网安备 11010802027423号
京公网安备 11010802027423号