当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Energy landscapes for the aggregation of Aβ17-42
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-02-27 , DOI: 10.1021/jacs.7b12896 Konstantin Röder 1 , David J. Wales 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-02-27 , DOI: 10.1021/jacs.7b12896 Konstantin Röder 1 , David J. Wales 1
Affiliation
|
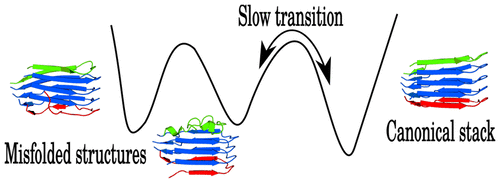
The aggregation of the Aβ peptide (Aβ1-42) to form fibrils is a key feature of Alzheimer's disease. The mechanism is thought to be a nucleation stage followed by an elongation process. The elongation stage involves the consecutive addition of monomers to one end of the growing fibril. The aggregation process proceeds in a stop-and-go fashion and may involve off-pathway aggregates, complicating experimental and computational studies. Here we present exploration of a well-defined region in the free and potential energy landscapes for the Aβ17-42 pentamer. We find that the ideal aggregation process agrees with the previously reported dock-lock mechanism. We also analyze a large number of additional stable structures located on the multifunnel energy landscape, which constitute kinetic traps. The key contributors to the formation of such traps are misaligned strong interactions, for example the stacking of F19 and F20, as well as entropic contributions. Our results suggest that folding templates for aggregation are a necessity and that aggregation studies could employ such species to obtain a more detailed description of the process.
中文翻译:

Aβ17-42 聚集的能量景观
Aβ 肽 (Aβ1-42) 聚集形成原纤维是阿尔茨海默病的一个关键特征。该机制被认为是一个成核阶段,然后是一个延伸过程。伸长阶段包括将单体连续添加到生长原纤维的一端。聚合过程以走走停停的方式进行,可能涉及路径外聚合,使实验和计算研究复杂化。在这里,我们展示了对 Aβ17-42 五聚体的自由和势能景观中明确定义的区域的探索。我们发现理想的聚合过程与之前报道的停靠锁定机制一致。我们还分析了位于多漏斗能量景观上的大量额外稳定结构,它们构成了动力学陷阱。形成这种陷阱的关键因素是未对齐的强相互作用,例如 F19 和 F20 的堆叠,以及熵的贡献。我们的结果表明,用于聚合的折叠模板是必要的,并且聚合研究可以利用此类物种来获得对过程的更详细描述。
更新日期:2018-02-27
中文翻译:

Aβ17-42 聚集的能量景观
Aβ 肽 (Aβ1-42) 聚集形成原纤维是阿尔茨海默病的一个关键特征。该机制被认为是一个成核阶段,然后是一个延伸过程。伸长阶段包括将单体连续添加到生长原纤维的一端。聚合过程以走走停停的方式进行,可能涉及路径外聚合,使实验和计算研究复杂化。在这里,我们展示了对 Aβ17-42 五聚体的自由和势能景观中明确定义的区域的探索。我们发现理想的聚合过程与之前报道的停靠锁定机制一致。我们还分析了位于多漏斗能量景观上的大量额外稳定结构,它们构成了动力学陷阱。形成这种陷阱的关键因素是未对齐的强相互作用,例如 F19 和 F20 的堆叠,以及熵的贡献。我们的结果表明,用于聚合的折叠模板是必要的,并且聚合研究可以利用此类物种来获得对过程的更详细描述。

























 京公网安备 11010802027423号
京公网安备 11010802027423号