当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Experimental and theoretical study of the inclusion complexes of epinephrine with β-cyclodextrin, 18-crown-6 and cucurbit[7]uril†
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2018-02-27 00:00:00 , DOI: 10.1039/c7nj04766e Suad K. S. Al-Burtomani 1, 2, 3, 4 , FakhrEldin O. Suliman 1, 2, 3, 4
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2018-02-27 00:00:00 , DOI: 10.1039/c7nj04766e Suad K. S. Al-Burtomani 1, 2, 3, 4 , FakhrEldin O. Suliman 1, 2, 3, 4
Affiliation
|
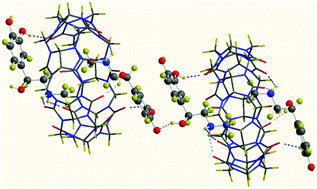
The binary and ternary complexes of epinephrine (EP) with β-cyclodextrin (βCD), 18-crown-6 (18C6) and cucurbit[7]uril (CB7) were probed using different experimental techniques. The results show that EP forms a stable binary complex with the three hosts. The fluorescence spectroscopy measurements showed enhancement in the emission spectrum at around 312 nm when βCD was added to the aqueous solution of EP, whereas the addition of CB7 and 18C6 caused quenching of this band accompanied by evolution of a band at around 412 nm. The 1H NMR spectroscopy confirmed the formation of the inclusion complexes. The 1H NMR spectroscopy indicated that the drug enters the CD nanocavity from its wider rim and deeply inserts the catechol moiety into the cavity. The association constants of binary complexes estimated by diffusion-ordered spectroscopy, DOSY, indicated a more stable complex was formed between EP and CB7. On the other hand, ESI-MS and MALDI-TOF data suggest that complexes of various stoichiometries are formed. This discloses the presence of binary and ternary complexes between EP and the three hosts. These data together with IR, Raman and PXRD for the lyophilized complexes clearly suggest that EP forms stable complexes with the three hosts in the solid and aqueous phase. These results were further clarified using molecular dynamics simulations for the five different complexes in aqueous media for 30 ns. The results obtained indicated that the hydrophobic effects and the hydrogen bonding interactions are the driving forces for the formation of the complexes and are responsible for their stabilities.
中文翻译:

肾上腺素与β-环糊精,18-crown-6和葫芦素[7] uril †的包合物的实验和理论研究
肾上腺素(EP)与β-环糊精(βCD),18-crown-6(18C6)和葫芦[7] uril(CB7)的二元和三元复合物使用不同的实验技术进行了探测。结果表明,EP与这三个宿主形成了稳定的二元复合物。荧光光谱测量显示,当将βCD添加到EP的水溶液中时,在312 nm处发射光谱增强,而CB7和18C6的添加导致该谱带淬灭,并伴随着412 nm处的谱带演化。的1个H NMR光谱证实了包合配合物的形成。在11 H NMR光谱表明该药物从其较宽的边缘进入CD纳米腔,并将邻苯二酚部分深深插入腔中。通过扩散有序光谱法DOSY估算的二元配合物的缔合常数表明,EP和CB7之间形成了更稳定的配合物。另一方面,ESI-MS和MALDI-TOF数据表明形成了各种化学计量的配合物。这公开了EP与三个主体之间存在二元和三元复合物。这些数据以及冻干配合物的IR,拉曼和PXRD清楚地表明,EP与固相和水相中的三种主体形成稳定的配合物。使用分子动力学模拟对水介质中的五种不同络合物进行30 ns的分析,可以进一步阐明这些结果。
更新日期:2018-02-27
中文翻译:

肾上腺素与β-环糊精,18-crown-6和葫芦素[7] uril †的包合物的实验和理论研究
肾上腺素(EP)与β-环糊精(βCD),18-crown-6(18C6)和葫芦[7] uril(CB7)的二元和三元复合物使用不同的实验技术进行了探测。结果表明,EP与这三个宿主形成了稳定的二元复合物。荧光光谱测量显示,当将βCD添加到EP的水溶液中时,在312 nm处发射光谱增强,而CB7和18C6的添加导致该谱带淬灭,并伴随着412 nm处的谱带演化。的1个H NMR光谱证实了包合配合物的形成。在11 H NMR光谱表明该药物从其较宽的边缘进入CD纳米腔,并将邻苯二酚部分深深插入腔中。通过扩散有序光谱法DOSY估算的二元配合物的缔合常数表明,EP和CB7之间形成了更稳定的配合物。另一方面,ESI-MS和MALDI-TOF数据表明形成了各种化学计量的配合物。这公开了EP与三个主体之间存在二元和三元复合物。这些数据以及冻干配合物的IR,拉曼和PXRD清楚地表明,EP与固相和水相中的三种主体形成稳定的配合物。使用分子动力学模拟对水介质中的五种不同络合物进行30 ns的分析,可以进一步阐明这些结果。


























 京公网安备 11010802027423号
京公网安备 11010802027423号