Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effect of Meropenem-Vaborbactam vs Piperacillin-Tazobactam on Clinical Cure or Improvement and Microbial Eradication in Complicated Urinary Tract Infection
JAMA ( IF 120.7 ) Pub Date : 2018-02-27 , DOI: 10.1001/jama.2018.0438 Keith S. Kaye 1 , Tanaya Bhowmick 2 , Symeon Metallidis 3 , Susan C. Bleasdale 4 , Olexiy S. Sagan 5 , Viktor Stus 6 , Jose Vazquez 7 , Valerii Zaitsev 8 , Mohamed Bidair 9 , Erik Chorvat 10 , Petru Octavian Dragoescu 11 , Elena Fedosiuk 12 , Juan P. Horcajada 13 , Claudia Murta 14 , Yaroslav Sarychev 15 , Ventsislav Stoev 16 , Elizabeth Morgan 17 , Karen Fusaro 18 , David Griffith 17 , Olga Lomovskaya 17 , Elizabeth L. Alexander 19 , Jeffery Loutit 17 , Michael N. Dudley 17 , Evangelos J. Giamarellos-Bourboulis 20
JAMA ( IF 120.7 ) Pub Date : 2018-02-27 , DOI: 10.1001/jama.2018.0438 Keith S. Kaye 1 , Tanaya Bhowmick 2 , Symeon Metallidis 3 , Susan C. Bleasdale 4 , Olexiy S. Sagan 5 , Viktor Stus 6 , Jose Vazquez 7 , Valerii Zaitsev 8 , Mohamed Bidair 9 , Erik Chorvat 10 , Petru Octavian Dragoescu 11 , Elena Fedosiuk 12 , Juan P. Horcajada 13 , Claudia Murta 14 , Yaroslav Sarychev 15 , Ventsislav Stoev 16 , Elizabeth Morgan 17 , Karen Fusaro 18 , David Griffith 17 , Olga Lomovskaya 17 , Elizabeth L. Alexander 19 , Jeffery Loutit 17 , Michael N. Dudley 17 , Evangelos J. Giamarellos-Bourboulis 20
Affiliation
|
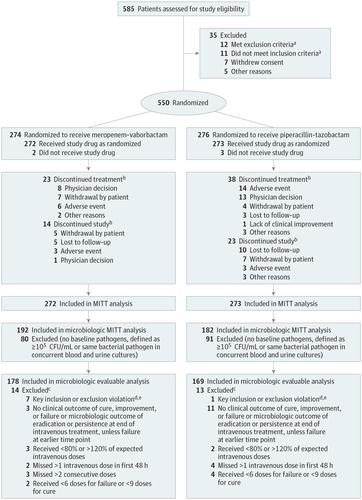
Importance Meropenem-vaborbactam is a combination carbapenem/beta-lactamase inhibitor and a potential treatment for severe drug-resistant gram-negative infections. Objective To evaluate efficacy and adverse events of meropenem-vaborbactam in complicated urinary tract infection (UTI), including acute pyelonephritis. Design, Setting, and Participants Phase 3, multicenter, multinational, randomized clinical trial (TANGO I) conducted November 2014 to April 2016 and enrolling patients (≥18 years) with complicated UTI, stratified by infection type and geographic region. Interventions Eligible patients were randomized 1:1 to receive meropenem-vaborbactam (2g/2g over 3 hours; n = 274) or piperacillin-tazobactam (4g/0.5g over 30 minutes; n = 276) every 8 hours. After 15 or more doses, patients could be switched to oral levofloxacin if they met prespecified criteria for improvement, to complete 10 days of total treatment. Main Outcomes and Measures Primary end point for FDA criteria was overall success (clinical cure or improvement and microbial eradication composite) at end of intravenous treatment in the microbiologic modified intent-to-treat (ITT) population. Primary end point for European Medicines Agency (EMA) criteria was microbial eradication at test-of-cure visit in the microbiologic modified ITT and microbiologic evaluable populations. Prespecified noninferiority margin was −15%. Because the protocol prespecified superiority testing in the event of noninferiority, 2-sided 95% CIs were calculated. Results Among 550 patients randomized, 545 received study drug (mean age, 52.8 years; 361 [66.2%] women; 374 [68.6%] in the microbiologic modified ITT population; 347 [63.7%] in the microbiologic evaluable population; 508 [93.2%] completed the trial). For the FDA primary end point, overall success occurred in 189 of 192 (98.4%) with meropenem-vaborbactam vs 171 of 182 (94.0%) with piperacillin-tazobactam (difference, 4.5% [95% CI, 0.7% to 9.1%]; P < .001 for noninferiority). For the EMA primary end point, microbial eradication in the microbiologic modified ITT population occurred in 128 of 192 (66.7%) with meropenem-vaborbactam vs 105 of 182 (57.7%) with piperacillin-tazobactam (difference, 9.0% [95% CI, −0.9% to 18.7%]; P < .001 for noninferiority); microbial eradication in the microbiologic evaluable population occurred in 118 of 178 (66.3%) vs 102 of 169 (60.4%) (difference, 5.9% [95% CI, −4.2% to 16.0%]; P < .001 for noninferiority). Adverse events were reported in 106 of 272 (39.0%) with meropenem-vaborbactam vs 97 of 273 (35.5%) with piperacillin-tazobactam. Conclusions and Relevance Among patients with complicated UTI, including acute pyelonephritis and growth of a baseline pathogen, meropenem-vaborbactam vs piperacillin-tazobactam resulted in a composite outcome of complete resolution or improvement of symptoms along with microbial eradication that met the noninferiority criterion. Further research is needed to understand the spectrum of patients in whom meropenem-vaborbactam offers a clinical advantage. Trial Registration clinicaltrials.gov Identifier: NCT02166476
中文翻译:

美罗培南-瓦波巴坦与哌拉西林-他唑巴坦对复杂尿路感染临床治愈或改善及微生物根除的影响
重要性 Meropenem-vaborbactam 是一种组合碳青霉烯/β-内酰胺酶抑制剂,可用于治疗严重的耐药革兰氏阴性菌感染。目的评价美罗培南-瓦硼巴坦治疗复杂性尿路感染(UTI),包括急性肾盂肾炎的疗效和不良反应。设计、设置和参与者 第 3 期多中心、跨国、随机临床试验 (TANGO I) 于 2014 年 11 月至 2016 年 4 月进行,招募了复杂性 UTI 患者(≥18 岁),按感染类型和地理区域分层。干预 符合条件的患者按 1:1 随机分组,每 8 小时接受美罗培南-瓦硼巴坦(3 小时内 2g/2g;n = 274)或哌拉西林-他唑巴坦(30 分钟内 4g/0.5g;n = 276)。服用 15 剂或更多剂后,如果患者符合预先指定的改善标准,则可以改用口服左氧氟沙星,以完成 10 天的总治疗。主要结果和测量 FDA 标准的主要终点是微生物学改良意向治疗 (ITT) 人群静脉治疗结束时的总体成功(临床治愈或改善和微生物根除复合)。欧洲药品管理局 (EMA) 标准的主要终点是微生物学改良 ITT 和微生物学可评估人群在治愈测试访视时的微生物根除。预设的非劣效性边际为-15%。由于方案预先指定了非劣效性事件中的优效性测试,因此计算了 2 侧 95% CI。结果 在随机分配的 550 名患者中,545 名接受了研究药物治疗(平均年龄 52.8 岁;361 名 [66.2%] 女性;374 [68. 6%] 在微生物改良的 ITT 人群中;347 [63.7%] 在微生物可评估人群中;508 [93.2%] 完成了试验)。对于 FDA 的主要终点,美罗培南-瓦硼巴坦组 192 例中的 189 例 (98.4%) 与哌拉西林-他唑巴坦组 182 例中的 171 例 (94.0%) 总体成功(差异,4.5% [95% CI,0.7% 至 9.1%] ; P < .001 为非劣效性)。对于 EMA 主要终点,使用美罗培南 - vaborbactam 的 192 名中的 128 名(66.7%)与使用哌拉西林 - 他唑巴坦的 182 名中的 105 名(57.7%)发生微生物根除(差异,9.0% [95% CI, −0.9% 至 18.7%];非劣效性 P < .001);微生物学可评估人群中的微生物根除发生在 178 人中的 118 人 (66.3%) 和 169 人中的 102 人 (60.4%)(差异,5.9% [95% CI,-4.2% 至 16.0%];非劣效性 P < .001)。美罗培南-瓦硼巴坦组 272 例中的 106 例(39.0%)报告了不良事件,哌拉西林-他唑巴坦组 273 例中的 97 例(35.5%)报告了不良事件。结论和相关性 在复杂性 UTI 患者中,包括急性肾盂肾炎和基线病原体的生长,美罗培南-瓦硼巴坦与哌拉西林-他唑巴坦导致症状完全消退或改善以及微生物根除的复合结果,符合非劣效性标准。需要进一步研究以了解美罗培南-瓦硼巴坦具有临床优势的患者范围。试验注册clinicaltrials.gov 标识符:NCT02166476 包括急性肾盂肾炎和基线病原体的生长,美罗培南-瓦硼巴坦与哌拉西林-他唑巴坦导致症状完全消退或改善以及微生物根除的复合结果,符合非劣效性标准。需要进一步研究以了解美罗培南-瓦硼巴坦具有临床优势的患者范围。试验注册clinicaltrials.gov 标识符:NCT02166476 包括急性肾盂肾炎和基线病原体的生长,美罗培南-瓦硼巴坦与哌拉西林-他唑巴坦导致症状完全消退或改善以及微生物根除的复合结果,符合非劣效性标准。需要进一步研究以了解美罗培南-瓦硼巴坦具有临床优势的患者范围。试验注册clinicaltrials.gov 标识符:NCT02166476
更新日期:2018-02-27
中文翻译:

美罗培南-瓦波巴坦与哌拉西林-他唑巴坦对复杂尿路感染临床治愈或改善及微生物根除的影响
重要性 Meropenem-vaborbactam 是一种组合碳青霉烯/β-内酰胺酶抑制剂,可用于治疗严重的耐药革兰氏阴性菌感染。目的评价美罗培南-瓦硼巴坦治疗复杂性尿路感染(UTI),包括急性肾盂肾炎的疗效和不良反应。设计、设置和参与者 第 3 期多中心、跨国、随机临床试验 (TANGO I) 于 2014 年 11 月至 2016 年 4 月进行,招募了复杂性 UTI 患者(≥18 岁),按感染类型和地理区域分层。干预 符合条件的患者按 1:1 随机分组,每 8 小时接受美罗培南-瓦硼巴坦(3 小时内 2g/2g;n = 274)或哌拉西林-他唑巴坦(30 分钟内 4g/0.5g;n = 276)。服用 15 剂或更多剂后,如果患者符合预先指定的改善标准,则可以改用口服左氧氟沙星,以完成 10 天的总治疗。主要结果和测量 FDA 标准的主要终点是微生物学改良意向治疗 (ITT) 人群静脉治疗结束时的总体成功(临床治愈或改善和微生物根除复合)。欧洲药品管理局 (EMA) 标准的主要终点是微生物学改良 ITT 和微生物学可评估人群在治愈测试访视时的微生物根除。预设的非劣效性边际为-15%。由于方案预先指定了非劣效性事件中的优效性测试,因此计算了 2 侧 95% CI。结果 在随机分配的 550 名患者中,545 名接受了研究药物治疗(平均年龄 52.8 岁;361 名 [66.2%] 女性;374 [68. 6%] 在微生物改良的 ITT 人群中;347 [63.7%] 在微生物可评估人群中;508 [93.2%] 完成了试验)。对于 FDA 的主要终点,美罗培南-瓦硼巴坦组 192 例中的 189 例 (98.4%) 与哌拉西林-他唑巴坦组 182 例中的 171 例 (94.0%) 总体成功(差异,4.5% [95% CI,0.7% 至 9.1%] ; P < .001 为非劣效性)。对于 EMA 主要终点,使用美罗培南 - vaborbactam 的 192 名中的 128 名(66.7%)与使用哌拉西林 - 他唑巴坦的 182 名中的 105 名(57.7%)发生微生物根除(差异,9.0% [95% CI, −0.9% 至 18.7%];非劣效性 P < .001);微生物学可评估人群中的微生物根除发生在 178 人中的 118 人 (66.3%) 和 169 人中的 102 人 (60.4%)(差异,5.9% [95% CI,-4.2% 至 16.0%];非劣效性 P < .001)。美罗培南-瓦硼巴坦组 272 例中的 106 例(39.0%)报告了不良事件,哌拉西林-他唑巴坦组 273 例中的 97 例(35.5%)报告了不良事件。结论和相关性 在复杂性 UTI 患者中,包括急性肾盂肾炎和基线病原体的生长,美罗培南-瓦硼巴坦与哌拉西林-他唑巴坦导致症状完全消退或改善以及微生物根除的复合结果,符合非劣效性标准。需要进一步研究以了解美罗培南-瓦硼巴坦具有临床优势的患者范围。试验注册clinicaltrials.gov 标识符:NCT02166476 包括急性肾盂肾炎和基线病原体的生长,美罗培南-瓦硼巴坦与哌拉西林-他唑巴坦导致症状完全消退或改善以及微生物根除的复合结果,符合非劣效性标准。需要进一步研究以了解美罗培南-瓦硼巴坦具有临床优势的患者范围。试验注册clinicaltrials.gov 标识符:NCT02166476 包括急性肾盂肾炎和基线病原体的生长,美罗培南-瓦硼巴坦与哌拉西林-他唑巴坦导致症状完全消退或改善以及微生物根除的复合结果,符合非劣效性标准。需要进一步研究以了解美罗培南-瓦硼巴坦具有临床优势的患者范围。试验注册clinicaltrials.gov 标识符:NCT02166476



























 京公网安备 11010802027423号
京公网安备 11010802027423号