PLOS Neglected Tropical Diseases ( IF 3.8 ) Pub Date : 2018-02-26 , DOI: 10.1371/journal.pntd.0006225 Valeska Albuquerque Francesconi , Fabio Francesconi , Rajendranath Ramasawmy , Gustavo Adolfo Sierra Romero , Maria das Graças Costa Alecrim
|
Background
The treatment of Leishmaniasis caused by Leishmania (Viannia) guyanensis is based on a weak strength of evidence from very few clinical trials and some case series reports. Current treatment guidelines recommend pentamidine isethionate or meglumine antimoniate (Glucantime) as the first-line choices. Both are parenteral drugs with a low therapeutic indexes leading to a high risk of undesired effects. Imidazole derivatives interfere with the production of leishmanial ergosterol, an essential component of their membrane structure. One drug that has been studied in different clinical presentations of Leishmania is fluconazole, a hydrophilic bis-triazole, which is easily absorbed through the oral route with a low toxicity profile and is considered safe for children. This drug is readily available in poor countries with a reasonable cost making it a potential option for treating leishmaniasis.
Methods and findings
An adaptive nonrandomized clinical trial with sequential groups with dose escalation of oral fluconazole was designed to treat adult men with localized cutaneous leishmaniasis (LCL) in Manaus, Brazil. Eligible participants were patients with LCL with confirmed Leishmania guyanensis infection.
Results
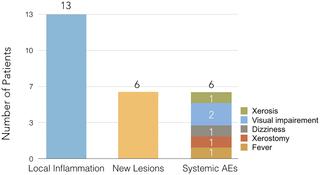
Twenty adult male patients were treated with 450 mg of fluconazole daily for 30 days. One patient (5%) was cured within 30 days of treatment. Of the 19 failures (95%), 13 developed a worsening of ulcers and six evolved lymphatic spreading of the disease. Planned dose escalation was suspended after the disappointing failure rate during the first stage of the trial.
Conclusion/Significance
Oral fluconazole, at the dose of 450mg per day, was not efficacious against LCL caused by Leishmania guyanensis in adult men.
Trial registration
Brazilian Clinical Trial Registration (ReBec)—RBR-8w292w; UTN number—1158-2421
中文翻译:

氟康唑未能治疗巴西亚马逊河中由圭亚那Leishmania Guyanensis引起的皮肤利什曼病:一项开放的,非随机的2期临床试验
背景
由Leishmania(Viannia)guyanensis引起的Leishmaniasis的治疗基于很少的临床试验和一些病例系列报告的证据不足。目前的治疗指南建议将戊tam乙磺酸乙磺酸盐或葡甲胺锑酸盐(Glucantime)作为首选药物。两者都是具有低治疗指数的肠胃外药物,导致产生不良作用的高风险。咪唑衍生物会干扰利什曼麦角固醇的产生,后者是其膜结构的重要组成部分。在利什曼原虫的不同临床表现中已研究的一种药物氟康唑是一种亲水性双三唑,它很容易通过口服途径被吸收,且毒性低,对儿童安全。这种药物在贫穷国家很容易买到,而且价格合理,使其成为治疗利什曼病的潜在选择。
方法和发现
一项适应性非随机临床试验,按顺序进行,并按顺序增加口服氟康唑的剂量,旨在治疗巴西马瑙斯地区的局部皮肤利什曼病(LCL)的成年男性。符合条件的参与者是确诊盖伊利什曼原虫感染的LCL患者。
结果
20名成年男性患者每天接受450 mg氟康唑治疗30天。一名患者(5%)在治疗后30天内治愈。在19例失败病例(95%)中,有13例溃疡恶化,有6例疾病淋巴扩散。在试验的第一阶段失败的失败率令人失望之后,暂停了计划的剂量递增。
结论/意义
口服氟康唑每天剂量为450mg,在成年男性中对由利什曼原虫Guyanensis引起的LCL无效。
试用注册
巴西临床试验注册(ReBec)-RBR-8w292w;UTN编号-1158-2421



























 京公网安备 11010802027423号
京公网安备 11010802027423号