当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
van der Waals interaction of HNCO and H2: Potential Energy Surface and Rotational Energy Transfer
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-02-26 00:00:00 , DOI: 10.1021/acs.jpca.8b00150 Emna Sahnoun 1, 2 , Laurent Wiesenfeld 1, 3 , Kamel Hammami 2 , Nejmeddine Jaidane 2
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-02-26 00:00:00 , DOI: 10.1021/acs.jpca.8b00150 Emna Sahnoun 1, 2 , Laurent Wiesenfeld 1, 3 , Kamel Hammami 2 , Nejmeddine Jaidane 2
Affiliation
|
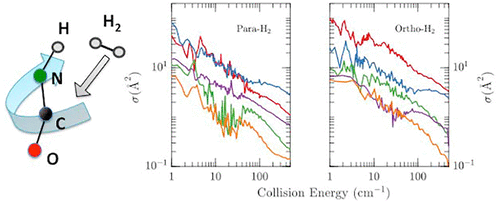
Isocyanic acid (HNCO) is the most stable of all its isomers; it has been observed repeatedly in many different conditions of the Interstellar Media, and its chemistry is poorly known. To quantitatively estimate the abundance of HNCO with respect to other organic molecules, we compute its rotational quenching rates colliding with H2, the most common gas in the gaseous Interstellar Media. We compute ab initio the van der Waals interaction HNCO–H2, in the rigid molecules approximation, with a CCSD(T)-F12a method. On the fitted ab initio surface, inelastic scattering cross sections and rates are calculated for a temperature range of 7–200 K, with the coupled-states quantum time-independent formalism. The critical densities are high enough to yield rotational temperatures of HNCO differing significantly from the kinetic temperature of H2, especially so for the shorter wavelengths observed at the ALMA interferometer. It is found that the quenching rates for collisions with ortho- or para-H2 differ greatly, opening the possibility of far from equilibrium populations of some rotational levels of HNCO.
中文翻译:

HNCO和H 2的van der Waals相互作用:势能表面和旋转能量转移
异氰酸(HNCO)是所有异构体中最稳定的;在星际媒体的许多不同条件下,人们反复观察到它的存在,但其化学性质却鲜为人知。为了定量估计HNCO相对于其他有机分子的丰度,我们计算了其旋转猝灭速率与H 2碰撞,H 2是气态星际介质中最常见的气体。我们使用CCSD(T)-F12a方法从刚性分子近似计算从头开始范德华相互作用HNCO–H 2。在合适的从头开始在7–200 K的温度范围内计算了表面,非弹性散射的横截面和速率,以及耦合态量子时间无关的形式主义。临界密度足够高,以致产生HNCO的旋转温度与H 2的动力学温度显着不同,尤其是对于在ALMA干涉仪上观察到的较短波长而言。发现与邻H 2或对H 2碰撞的猝灭速率相差很大,这为远离某些旋转水平的HNCO的平衡族提供了可能性。
更新日期:2018-02-26
中文翻译:

HNCO和H 2的van der Waals相互作用:势能表面和旋转能量转移
异氰酸(HNCO)是所有异构体中最稳定的;在星际媒体的许多不同条件下,人们反复观察到它的存在,但其化学性质却鲜为人知。为了定量估计HNCO相对于其他有机分子的丰度,我们计算了其旋转猝灭速率与H 2碰撞,H 2是气态星际介质中最常见的气体。我们使用CCSD(T)-F12a方法从刚性分子近似计算从头开始范德华相互作用HNCO–H 2。在合适的从头开始在7–200 K的温度范围内计算了表面,非弹性散射的横截面和速率,以及耦合态量子时间无关的形式主义。临界密度足够高,以致产生HNCO的旋转温度与H 2的动力学温度显着不同,尤其是对于在ALMA干涉仪上观察到的较短波长而言。发现与邻H 2或对H 2碰撞的猝灭速率相差很大,这为远离某些旋转水平的HNCO的平衡族提供了可能性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号