当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Programmed Protein Self-Assembly Driven by Genetically Encoded Intein-Mediated Native Chemical Ligation
ACS Synthetic Biology ( IF 4.7 ) Pub Date : 2018-02-23 00:00:00 , DOI: 10.1021/acssynbio.7b00447 Joseph A Harvey 1 , Laura S Itzhaki 2 , Ewan R G Main 1
ACS Synthetic Biology ( IF 4.7 ) Pub Date : 2018-02-23 00:00:00 , DOI: 10.1021/acssynbio.7b00447 Joseph A Harvey 1 , Laura S Itzhaki 2 , Ewan R G Main 1
Affiliation
|
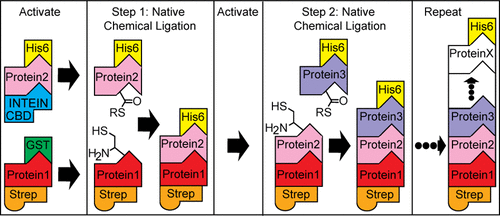
Harnessing and controlling self-assembly is an important step in developing proteins as novel biomaterials. With this goal, here we report the design of a general genetically programmed system that covalently concatenates multiple distinct protein domains into specific assembled arrays. It is driven by iterative intein-mediated native chemical ligation (NCL) under mild native conditions. The system uses a series of initially inert recombinant protein fusions that sandwich the protein modules to be ligated between one of a number of different affinity tags and an intein protein domain. Orthogonal activation at opposite termini of compatible protein fusions, via protease and intein cleavage, coupled with sequential mixing directs an irreversible and traceless stepwise assembly process. This gives total control over the composition and arrangement of component proteins within the final product, enabled the limits of the system—reaction efficiency and yield—to be investigated, and led to the production of “functional” assemblies.
中文翻译:

由基因编码的内含肽介导的天然化学连接驱动的程序化蛋白质自组装
利用和控制自组装是开发蛋白质作为新型生物材料的重要一步。带着这个目标,我们在这里报告了一个通用基因编程系统的设计,该系统将多个不同的蛋白质结构域共价连接到特定的组装阵列中。它是在温和的天然条件下由迭代内含肽介导的天然化学连接 (NCL) 驱动的。该系统使用一系列最初惰性的重组蛋白融合体,将待连接的蛋白质模块夹在多个不同的亲和标签之一和内含肽结构域之间。通过蛋白酶和内含肽切割,在相容蛋白融合的相反末端进行正交激活,再加上顺序混合,指导了一个不可逆和无痕的逐步组装过程。
更新日期:2018-02-23
中文翻译:

由基因编码的内含肽介导的天然化学连接驱动的程序化蛋白质自组装
利用和控制自组装是开发蛋白质作为新型生物材料的重要一步。带着这个目标,我们在这里报告了一个通用基因编程系统的设计,该系统将多个不同的蛋白质结构域共价连接到特定的组装阵列中。它是在温和的天然条件下由迭代内含肽介导的天然化学连接 (NCL) 驱动的。该系统使用一系列最初惰性的重组蛋白融合体,将待连接的蛋白质模块夹在多个不同的亲和标签之一和内含肽结构域之间。通过蛋白酶和内含肽切割,在相容蛋白融合的相反末端进行正交激活,再加上顺序混合,指导了一个不可逆和无痕的逐步组装过程。



























 京公网安备 11010802027423号
京公网安备 11010802027423号