当前位置:
X-MOL 学术
›
Chem. Eng. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hydrogen storage properties of nanoconfined aluminium hydride (AlH 3 )
Chemical Engineering Science ( IF 4.7 ) Pub Date : 2019-02-01 , DOI: 10.1016/j.ces.2018.02.014 Lei Wang , Aditya Rawal , Kondo-Francois Aguey-Zinsou
Chemical Engineering Science ( IF 4.7 ) Pub Date : 2019-02-01 , DOI: 10.1016/j.ces.2018.02.014 Lei Wang , Aditya Rawal , Kondo-Francois Aguey-Zinsou
|
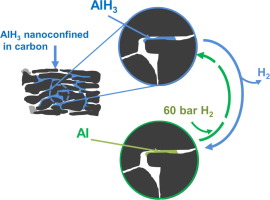
Abstract AlH 3 (alane) is considered as an ideal one-off compact hydrogen storage material thanks to its high storage capacity and moderate decomposition temperature. However, application has been hindered by the lack of direct hydrogen reversibility, i.e. regeneration of AlH 3 directly from the reaction of spent Al with hydrogen under mild conditions. Herein, we investigated the effect of nanoconfinement on the properties of AlH 3 and the potential of this approach toward the direct release and uptake of hydrogen by nanosized Al. AlH 3 was confined within the mesopores of a high surface area graphite (HSAG). The decomposition of the nanoconfined AlH 3 was detected 50 °C lower than the bulk, showing some effect of nanoconfinement on the overall temperature for hydrogen release. Some hydrogen uptake was also observed at 150 °C and 60 bar hydrogen pressure, and this indicates that reversible release of hydrogen with alane under moderate conditions of pressure and temperature may be possible.
中文翻译:

纳米限界氢化铝(AlH 3 )的储氢性能
摘要 AlH 3 (alane)由于其高存储容量和适中的分解温度被认为是一种理想的一次性致密储氢材料。然而,由于缺乏直接的氢可逆性,即在温和条件下从废铝与氢的反应直接再生AlH 3 ,应用受到了阻碍。在此,我们研究了纳米限制对 AlH 3 性质的影响以及这种方法对纳米尺寸 Al 直接释放和吸收氢的潜力。AlH 3 被限制在高表面积石墨(HSAG)的介孔内。检测到纳米限制 AlH 3 的分解比本体低 50°C,表明纳米限制对氢气释放的整体温度有一些影响。
更新日期:2019-02-01
中文翻译:

纳米限界氢化铝(AlH 3 )的储氢性能
摘要 AlH 3 (alane)由于其高存储容量和适中的分解温度被认为是一种理想的一次性致密储氢材料。然而,由于缺乏直接的氢可逆性,即在温和条件下从废铝与氢的反应直接再生AlH 3 ,应用受到了阻碍。在此,我们研究了纳米限制对 AlH 3 性质的影响以及这种方法对纳米尺寸 Al 直接释放和吸收氢的潜力。AlH 3 被限制在高表面积石墨(HSAG)的介孔内。检测到纳米限制 AlH 3 的分解比本体低 50°C,表明纳米限制对氢气释放的整体温度有一些影响。


























 京公网安备 11010802027423号
京公网安备 11010802027423号