Molecular Catalysis ( IF 4.6 ) Pub Date : 2018-02-22 , DOI: 10.1016/j.mcat.2018.01.037 Jifan Li , Yifeng Hou , Zhe Song , Chunling Liu , Wensheng Dong , Chenghua Zhang , Yong Yang , Yongwang Li
|
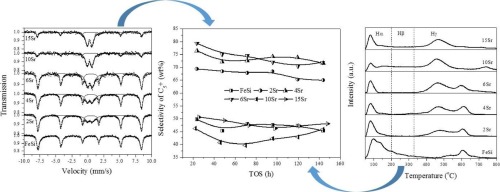
A series of Fe/SiO2 catalysts with different strontium/iron molar ratios were used to investigate the chemical and structural effects of strontium on the iron-based Fischer-Tropsch synthesis (FTS) catalysts. It was found that the chemical effect of strontium strengthened FeO bonds of Fe2O3 and hence suppressed the reduction of iron oxide. The chemical effect also suppressed the H2 adsorption but improved the CO adsorption, which is responsible for the low surface H/C ratios and methane selectivity. At the same time, the promotion of strontium improved the dispersion of iron oxides. The dispersion effect facilitated the reduction and carburization of catalysts. It was also helpful for the H2 adsorption and in turn led to high surface H/C ratios and methane selectivity. Under the combined action of the chemical and structural effects, the selectivity to methane of catalysts exhibited a parabola-like trend as a function of strontium loading and passed through a minimum at Sr/Fe molar ratio of 6/100. In contrast, the selectivity of heavy hydrocarbon demonstrated an opposite tendency.
中文翻译:

锶对铁基费托合成催化剂的化学和结构作用
一系列具有不同锶/铁摩尔比的Fe / SiO 2催化剂用于研究锶对铁基费-托合成(FTS)催化剂的化学和结构作用。发现锶的化学作用增强了Fe 2 O 3的Fe O键,从而抑制了氧化铁的还原。化学作用还抑制了H 2吸附,但改善了CO吸附,这是低表面H / C比和甲烷选择性的原因。同时,锶的促进改善了氧化铁的分散。分散作用促进了催化剂的还原和渗碳。对H 2也有帮助吸附,进而导致高的表面H / C比和甲烷选择性。在化学和结构效应的共同作用下,催化剂对甲烷的选择性呈现出类似锶的抛物线趋势,这是锶负载的函数,并且在Sr / Fe摩尔比为6/100时达到最小值。相反,重烃的选择性显示出相反的趋势。


























 京公网安备 11010802027423号
京公网安备 11010802027423号