当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Biological activities of 3,4,5‐trihydroxypiperidines and their N‐ and O‐derivatives
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2018-04-16 , DOI: 10.1111/cbdd.13182 Kate Prichard 1, 2 , David Campkin 1, 2 , Nicholas O'Brien 1, 2 , Atsushi Kato 3 , George W. J. Fleet 4 , Michela I. Simone 1, 2
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2018-04-16 , DOI: 10.1111/cbdd.13182 Kate Prichard 1, 2 , David Campkin 1, 2 , Nicholas O'Brien 1, 2 , Atsushi Kato 3 , George W. J. Fleet 4 , Michela I. Simone 1, 2
Affiliation
|
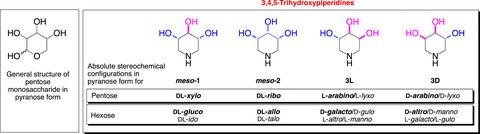
3,4,5‐Trihydroxypiperidines belong to the family of 1,5‐dideoxy‐1,5‐iminosugar natural products and are structural analogues of pentose monosaccharides in the pyranose form. The biological activities of these apparently structurally simple molecules and their N‐ and O‐alkylated and ‐arylated derivatives are no less remarkable than their C‐6 hydroxymethyl counterparts of the hexoses (such as 1‐deoxynojirimycin, DNJ). Their biological profiles indicate that the hydroxymethyl branch is crucial to neither potency nor selectivity, with O‐alkylation demonstrated to produce exquisite selectivity extending beyond glycosidase inhibition, to immunosuppressant and antibacterial activities.
中文翻译:

3,4,5-三羟基哌啶及其N和O衍生物的生物活性
3,4,5-三羟基哌啶属于1,5-二脱氧-1,5-亚氨基糖天然产物家族,是吡喃糖形式的戊糖单糖的结构类似物。这些看似结构简单的分子及其N-和O-烷基化和芳基化衍生物的生物活性不亚于己糖的C-6羟甲基对应物(例如1-deoxynojirimycin,DNJ)。它们的生物学特性表明,羟甲基支链对效能和选择性都不是至关重要的,O-烷基化已证明可产生精妙的选择性,其范围不仅限于糖苷酶抑制作用,还包括免疫抑制和抗菌活性。
更新日期:2018-04-16
中文翻译:

3,4,5-三羟基哌啶及其N和O衍生物的生物活性
3,4,5-三羟基哌啶属于1,5-二脱氧-1,5-亚氨基糖天然产物家族,是吡喃糖形式的戊糖单糖的结构类似物。这些看似结构简单的分子及其N-和O-烷基化和芳基化衍生物的生物活性不亚于己糖的C-6羟甲基对应物(例如1-deoxynojirimycin,DNJ)。它们的生物学特性表明,羟甲基支链对效能和选择性都不是至关重要的,O-烷基化已证明可产生精妙的选择性,其范围不仅限于糖苷酶抑制作用,还包括免疫抑制和抗菌活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号