当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exploring the Conversion of a d‐Sialic Acid Aldolase into a l‐KDO Aldolase
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2018-02-22 , DOI: 10.1002/ejoc.201800103 Adrian Romero-Rivera 1 , Javier Iglesias-Fernández 1 , Sílvia Osuna 1, 2
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2018-02-22 , DOI: 10.1002/ejoc.201800103 Adrian Romero-Rivera 1 , Javier Iglesias-Fernández 1 , Sílvia Osuna 1, 2
Affiliation
|
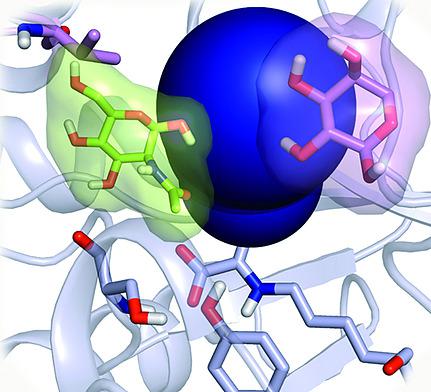
Directed evolution involves iterative rounds of genetic diversification followed by selection of variants with improved enzyme properties. A common trend in these studies is the introduction of multiple scattered mutations remote from the active site. Rationalizing how distal mutations influence the conformational states or ensemble of conformations formed by enzymes, and its relation to enzymatic catalysis is highly appealing for the improvement of current rational enzyme design strategies. Directed evolution was applied to convert d‐sialic acid aldolase into an efficient l‐3‐deoxy‐manno‐2‐octulosonic acid (l‐KDO) aldolase. In this study, we computationally evaluate the conformational dynamics of both enzymes to shed light on the specificity of the evolved variant. We further demonstrate the role of distal mutations on the modulation of enzyme conformational dynamics and its relation to substrate accessibility and selectivity. Mutations markedly altered the active site shape and substrate access tunnels in the evolved l‐KDO aldolase, thus affecting the enzyme specificity.
中文翻译:

探索将d-唾液酸醛缩酶转变为l- KDO醛缩酶
定向进化涉及遗传多样性的迭代轮次,然后选择具有改善的酶特性的变体。这些研究的共同趋势是在远离活动位点的地方引入了多个分散的突变。合理化远端突变如何影响酶形成的构象状态或构象集合及其与酶催化的关系,对于改进当前合理的酶设计策略具有极大的吸引力。定向进化应用于转换d -唾液酸醛缩酶成一个高效升-3-脱氧甘露-2-辛酮糖酸(升‐KDO)醛缩酶。在这项研究中,我们以计算方式评估了这两种酶的构象动力学,以阐明进化变体的特异性。我们进一步证明了远端突变对酶构象动力学的调节作用及其与底物可及性和选择性的关系。突变显着改变了进化的l- KDO醛缩酶中的活性位点形状和底物通道,从而影响了酶的特异性。
更新日期:2018-05-15
中文翻译:

探索将d-唾液酸醛缩酶转变为l- KDO醛缩酶
定向进化涉及遗传多样性的迭代轮次,然后选择具有改善的酶特性的变体。这些研究的共同趋势是在远离活动位点的地方引入了多个分散的突变。合理化远端突变如何影响酶形成的构象状态或构象集合及其与酶催化的关系,对于改进当前合理的酶设计策略具有极大的吸引力。定向进化应用于转换d -唾液酸醛缩酶成一个高效升-3-脱氧甘露-2-辛酮糖酸(升‐KDO)醛缩酶。在这项研究中,我们以计算方式评估了这两种酶的构象动力学,以阐明进化变体的特异性。我们进一步证明了远端突变对酶构象动力学的调节作用及其与底物可及性和选择性的关系。突变显着改变了进化的l- KDO醛缩酶中的活性位点形状和底物通道,从而影响了酶的特异性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号