Journal of Catalysis ( IF 7.3 ) Pub Date : 2018-02-22 , DOI: 10.1016/j.jcat.2018.01.032 Stanley Herrmann , Enrique Iglesia
|
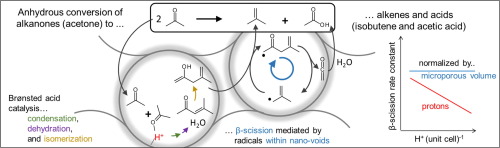
Solid Brønsted acids catalyze aldol condensations that form CC bonds and remove O-atoms from oxygenate reactants, but sequential β-scission reactions also cleave C
C bonds, leading to isobutene and acetic acid products for acetone reactants. The elementary steps and site requirements that cause these selectivities to depend sensitively on Al content and framework type in aluminosilicate solid acids remain speculative. Acetone reactions on microporous and mesoporous aluminosilicates (FER, TON, MFI, BEA, MCM-41) showed highest β-scission selectivities on MFI and BEA; they increased as the Al content and the intracrystalline density of active protons decreased. The effects of acetone and H2O pressure on turnover rates and selectivities indicate that an equilibrated pool of reactive C6 ketols and alkenones are present at pseudo-steady-state concentrations during catalysis and that they act as intermediates in β-scission routes. Two distinct C6 β-scission pathways contribute to the formation of isobutene and acetic acid: (i) a minor H2O-mediated route involving β-scission of C6 ketols on protons and (ii) the predominant anhydrous path, in which H-transfer forms unsaturated C6 enols at protons and these enols propagate radical chains mediated by transition states stabilized by van der Waals contacts within vicinal microporous voids. This latter route is consistent with coupled-cluster free energy estimates for these unsaturated C6 enols and their respective free radicals. It accounts for β-scission selectivities that increase with decreasing proton density, a finding that precludes the sole involvement of acid sites and requires instead the kinetic coupling between reactions at protons and propagation steps mediated by transition states confined within proximate voids, even when such voids lack a specific binding site. These mechanistic interpretations also account for the observed effects of residence time, of the loss of active protons by deactivation, of acetone and H2O pressures, and of aluminosilicate framework structure on selectivity. These mechanistic insights also demonstrate the ability of voids to stabilize transition states that mediate homogeneous reactions by mere confinement, even in the absence of chemical binding onto specific sites, as well as the essential requirement of intimate proximity for the effective kinetic coupling between reactions on protons and in proton-free voids, a process mediated by the diffusion of very reactive and unstable intermediates present at very low local concentrations within microporous frameworks.
中文翻译:

铝硅酸盐上丙酮到异丁烯和乙酸的选择性转化:酸催化和自由基介导的途径之间的动力学偶联
固体布朗斯台德酸催化形成C C键的醛醇缩合并从含氧化合物中除去O原子,但是连续的β断裂反应也裂解C
C键,从而生成用于丙酮反应物的异丁烯和乙酸产物。导致这些选择性敏感地取决于铝硅酸盐固体酸中Al含量和骨架类型的基本步骤和位置要求仍然是推测性的。在微孔和中孔铝硅酸盐(FER,TON,MFI,BEA,MCM-41)上的丙酮反应在MFI和BEA上表现出最高的β断裂选择性;它们随着铝含量的增加和活性质子晶体内密度的降低而增加。丙酮和H 2 O压力对周转率和选择性的影响表明反应性C的平衡池6吨酮醇以及烯酮存在于伪稳态浓度催化期间,并且它们充当β-断裂途径的中间体。两个不同的C- 6 β-断裂途径有助于异丁烯和乙酸的形成:(ⅰ)一个次要ħ 2 O形介导的路线涉及的Cβ-断裂6吨酮醇上的质子和(ii)的主要无水路径,其中H-转移在质子上形成不饱和C 6烯醇,这些烯醇传播由邻态微孔空隙内的范德华接触稳定的过渡态所介导的自由基链。后一条路线与这些不饱和C 6的耦合簇自由能估计相符烯醇及其各自的自由基。它解释了随着质子密度的降低而增加的β断裂选择性,这一发现排除了酸性位点的唯一参与,而是需要质子反应和由受限于邻近空隙内的过渡态介导的传播步骤之间的动力学耦合,即使这种空隙也是如此。缺乏特定的结合位点。这些机理的解释还解释了所观察到的停留时间,丙酮失活和氢2的失活引起的活性质子损失的影响。O压力和铝硅酸盐骨架结构对选择性的影响。这些机制的见解还表明,即使在没有化学键合到特定位点的情况下,空隙也能够稳定过渡状态,从而仅通过限制即可介导均相反应,并且对于质子上的反应之间的有效动力学偶联,必须紧密接近。在无质子的空隙中,是由微孔骨架内以非常低的局部浓度存在的非常活泼和不稳定的中间体的扩散所介导的过程。



























 京公网安备 11010802027423号
京公网安备 11010802027423号