Journal of Catalysis ( IF 7.3 ) Pub Date : 2018-02-22 , DOI: 10.1016/j.jcat.2018.01.007 Joseph B. Sweeney , Kirsty Adams , Julien Doulcet , Bimod Thapa , Fanny Tran , Robert Crook
|
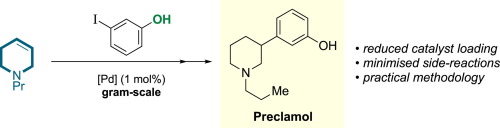
Though a widely used metal-catalyzed cross-coupling process, the Mizoroki–Heck (MH) reaction can be a capricious transformation. This is particularly true for oxidation-prone alkene substrates containing ligating heteroatoms, as in the case of N-alkyl tetrahydropyridines, whose MH reactions have been underexplored due to the many side reactions that hamper the process. Since the products of tetrahydropyridine Heck reactions are direct precursors to potent pharmacophores, and therefore of commercial value, this is a significant drawback. We report here the results of our study designed to deliver an optimized, scalable MH procedure for N-alkyltetrahydropyridines and its exemplification in a gram-scale synthesis of the drug substance preclamol.
中文翻译:

优化环状烯丙基胺的Mizoroki-Heck反应:无保护基团的克来克莫克的克兰级合成
尽管广泛使用了金属催化的交叉偶联过程,但Mizoroki-Heck(MH)反应可能是反复无常的转变。对于含有连接杂原子的易氧化烯烃底物尤其如此,例如在N-烷基四氢吡啶的情况下,由于许多阻碍反应的副反应,其MH反应尚未得到充分开发。由于四氢吡啶Heck反应的产物是有效药效团的直接前体,因此具有商业价值,因此这是一个重大缺陷。我们在这里报告了我们的研究结果,旨在为N-烷基四氢吡啶类化合物提供优化的,可扩展的MH程序及其在克雷克莫尔的克级合成中的例证。


























 京公网安备 11010802027423号
京公网安备 11010802027423号