Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Gold nanoparticles supported on mesoporous iron oxide for enhanced CO oxidation reaction
Nanoscale ( IF 6.7 ) Pub Date : 2018-02-22 00:00:00 , DOI: 10.1039/c7nr08895g Shunsuke Tanaka 1, 2, 3, 4 , Jianjian Lin 5, 6, 7, 8, 9 , Yusuf Valentino Kaneti 10, 11, 12, 13 , Shin-ichi Yusa 13, 14, 15, 16 , Yohei Jikihara 13, 17, 18 , Tsuruo Nakayama 13, 17, 18 , Mohamed Barakat Zakaria 10, 11, 12, 13, 19 , Abdulmohsen Ali Alshehri 19, 20, 21, 22 , Jungmok You 23, 24, 25, 26 , Md. Shahriar A. Hossain 1, 2, 3, 4 , Yusuke Yamauchi 23, 24, 25, 26, 27
Nanoscale ( IF 6.7 ) Pub Date : 2018-02-22 00:00:00 , DOI: 10.1039/c7nr08895g Shunsuke Tanaka 1, 2, 3, 4 , Jianjian Lin 5, 6, 7, 8, 9 , Yusuf Valentino Kaneti 10, 11, 12, 13 , Shin-ichi Yusa 13, 14, 15, 16 , Yohei Jikihara 13, 17, 18 , Tsuruo Nakayama 13, 17, 18 , Mohamed Barakat Zakaria 10, 11, 12, 13, 19 , Abdulmohsen Ali Alshehri 19, 20, 21, 22 , Jungmok You 23, 24, 25, 26 , Md. Shahriar A. Hossain 1, 2, 3, 4 , Yusuke Yamauchi 23, 24, 25, 26, 27
Affiliation
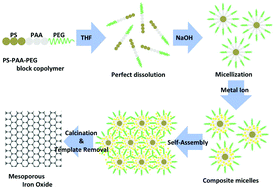
|
Herein, we report the synthesis of gold (Au)-loaded mesoporous iron oxide (Fe2O3) as a catalyst for both CO and NH3 oxidation. The mesoporous Fe2O3 is firstly prepared using polymeric micelles made of an asymmetric triblock copolymer poly(styrene-b-acrylic acid-b-ethylene glycol) (PS-b-PAA-b-PEG). Owing to its unique porous structure and large surface area (87.0 m2 g−1), the as-prepared mesoporous Fe2O3 can be loaded with a considerably higher amount of Au nanoparticles (Au NPs) (7.9 wt%) compared to the commercial Fe2O3 powder (0.8 wt%). Following the Au loading, the mesoporous Fe2O3 structure is still well-retained and Au NPs with varying sizes of 3–10 nm are dispersed throughout the mesoporous support. When evaluated for CO oxidation, the Au-loaded mesoporous Fe2O3 catalyst shows up to 20% higher CO conversion efficiency compared to the commercial Au/Fe2O3 catalyst, especially at lower temperatures (25–150 °C), suggesting the promising potential of this catalyst for low-temperature CO oxidation. Furthermore, the Au-loaded mesoporous Fe2O3 catalyst also displays a higher catalytic activity for NH3 oxidation with a respectable conversion efficiency of 37.4% compared to the commercial Au/Fe2O3 catalyst (15.6%) at 200 °C. The significant enhancement in the catalytic performance of the Au-loaded mesoporous Fe2O3 catalyst for both CO and NH3 oxidation may be attributed to the improved dispersion of the Au NPs and enhanced diffusivity of the reactant molecules due to the presence of mesopores and a higher oxygen activation rate contributed by the increased number of active sites, respectively.
中文翻译:

负载在介孔氧化铁上的金纳米颗粒可增强CO氧化反应
在本文中,我们报道了负载金(Au)的中孔氧化铁(Fe 2 O 3)的合成,该催化剂可同时催化CO和NH 3氧化。中孔的Fe 2 ö 3是使用由不对称三嵌段共聚物的聚(苯乙烯-聚合物胶束首先制备b -丙烯酸酸- b -亚乙基二醇)(PS- b -PAA- b -PEG)。由于其独特的多孔结构和较大的表面积(87.0 m 2 g -1),因此制得的介孔Fe 2 O 3与市售的Fe 2 O 3粉末(0.8重量%)相比,可向其负载明显更高量的金纳米颗粒(Au NP)(7.9重量%)。在Au负载后,介孔Fe 2 O 3结构仍然保持良好,并且大小在3–10 nm的Au NPs分散在整个介孔载体中。当评估CO氧化时,载有Au的中孔Fe 2 O 3催化剂与商业化的Au / Fe 2 O 3相比,显示出高达20%的CO转化效率催化剂,特别是在较低的温度(25–150°C)下,表明该催化剂在低温CO氧化方面的潜力很大。此外,与市售Au / Fe 2 O 3催化剂(15.6%)在200℃相比,Au负载的中孔Fe 2 O 3催化剂还显示出更高的NH 3氧化催化活性,转化效率为37.4%。负载金的介孔Fe 2 O 3催化剂对CO和NH 3的催化性能显着增强 氧化可以归因于由于中孔的存在而导致的Au NPs分散性的改善和反应物分子扩散性的提高,以及由于活性位点数量的增加而导致的较高的氧活化率。
更新日期:2018-02-22
中文翻译:

负载在介孔氧化铁上的金纳米颗粒可增强CO氧化反应
在本文中,我们报道了负载金(Au)的中孔氧化铁(Fe 2 O 3)的合成,该催化剂可同时催化CO和NH 3氧化。中孔的Fe 2 ö 3是使用由不对称三嵌段共聚物的聚(苯乙烯-聚合物胶束首先制备b -丙烯酸酸- b -亚乙基二醇)(PS- b -PAA- b -PEG)。由于其独特的多孔结构和较大的表面积(87.0 m 2 g -1),因此制得的介孔Fe 2 O 3与市售的Fe 2 O 3粉末(0.8重量%)相比,可向其负载明显更高量的金纳米颗粒(Au NP)(7.9重量%)。在Au负载后,介孔Fe 2 O 3结构仍然保持良好,并且大小在3–10 nm的Au NPs分散在整个介孔载体中。当评估CO氧化时,载有Au的中孔Fe 2 O 3催化剂与商业化的Au / Fe 2 O 3相比,显示出高达20%的CO转化效率催化剂,特别是在较低的温度(25–150°C)下,表明该催化剂在低温CO氧化方面的潜力很大。此外,与市售Au / Fe 2 O 3催化剂(15.6%)在200℃相比,Au负载的中孔Fe 2 O 3催化剂还显示出更高的NH 3氧化催化活性,转化效率为37.4%。负载金的介孔Fe 2 O 3催化剂对CO和NH 3的催化性能显着增强 氧化可以归因于由于中孔的存在而导致的Au NPs分散性的改善和反应物分子扩散性的提高,以及由于活性位点数量的增加而导致的较高的氧活化率。


























 京公网安备 11010802027423号
京公网安备 11010802027423号