Molecular Catalysis ( IF 4.6 ) Pub Date : 2018-02-21 , DOI: 10.1016/j.mcat.2018.01.017 Lulu Chen , Weiyu Song , Meizan Jing , Huiling Zheng , Jian Liu , Zhen Zhao , Zhongcheng Li
|
Highlights
- •
Show HCHO oxidation mechanism on both stoichiometric and defective Mn-doped ceria (110) surface.
- •
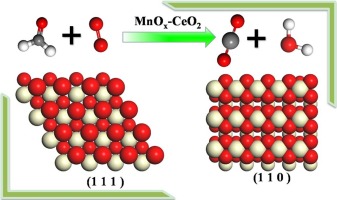
Clarify the influence of surface termination on Mn-doped ceria (110) and (111).
- •
State the formation and function of oxygen vacancy.
- •
Discuss the influence of different oxygen species.
Abstract
Mn-doped ceria is a highly efficient catalyst for HCHO oxidation. The activity is shown to be influenced strongly by the synthesis method, which could generate catalyst with different morphology. Density Functional Theory (DFT) was performed to gain insight into such surface termination effect. The mechanisms of HCHO oxidation by Mn-doped ceria (110) were extensively explored. The potential energy diagram will be compared with that on MnCe1-xO2(111). On the stoichiometric surface, HCHO oxidation followed a Mars-Van Krevelen mechanism. The main steps include HCHO adsorption, two C
 H bond cleavage, the activation energy barrier decreased sharply on MnCe1-xO2(110) as compared with that on MnCe1-xO2(111), which was due to smaller distortion for transition state and smaller distance of H to lattice O atom. A Langmuir-Hinshelwood mechanism was preferred on the defective surface due to the co-adsorption of HCHO and O2. Two C
H bond cleavage, the activation energy barrier decreased sharply on MnCe1-xO2(110) as compared with that on MnCe1-xO2(111), which was due to smaller distortion for transition state and smaller distance of H to lattice O atom. A Langmuir-Hinshelwood mechanism was preferred on the defective surface due to the co-adsorption of HCHO and O2. Two C H bonds of HCOH dissociated and the dissociated H atoms were adopted by adsorbed O2 species. Accordingly, similar activation barriers on these two defective surface were observed due to same oxidizing species. The present contribution provides a molecular level insight into the role of surface termination of ceria in HCHO oxidation, enabling further experimental improvement.
H bonds of HCOH dissociated and the dissociated H atoms were adopted by adsorbed O2 species. Accordingly, similar activation barriers on these two defective surface were observed due to same oxidizing species. The present contribution provides a molecular level insight into the role of surface termination of ceria in HCHO oxidation, enabling further experimental improvement.
中文翻译:

锰掺杂二氧化铈表面终止对甲醛氧化的影响:密度泛函理论研究
强调
- •
在化学计量的和有缺陷的Mn掺杂的二氧化铈(110)表面上均显示HCHO氧化机理。
- •
弄清表面终止对掺杂锰的二氧化铈(110)和(111)的影响。
- •
说明氧气空位的形成和功能。
- •
讨论不同氧气种类的影响。
抽象的
锰掺杂的二氧化铈是用于HCHO氧化的高效催化剂。活性受到合成方法的强烈影响,可以生成不同形态的催化剂。进行密度泛函理论(DFT)可以深入了解这种表面终止效应。Mn掺杂的二氧化铈(110)对HCHO氧化的机理进行了广泛的探索。势能图将与MnCe 1-x O 2(111)上的势能图进行比较。在化学计量表面上,HCHO氧化遵循Mars-Van Krevelen机理。主要步骤包括HCHO吸附,两个C
 H键裂解,激活能垒急剧下降上MnCe 1-x Ö 2(110),其与该上MnCe比较1-x Ö 2(111),这是由于对过渡状态小失真及H更小的距离,以晶格O原子。由于HCHO和O 2的共同吸附,在有缺陷的表面上优选使用Langmuir-Hinshelwood机理。两个C
H键裂解,激活能垒急剧下降上MnCe 1-x Ö 2(110),其与该上MnCe比较1-x Ö 2(111),这是由于对过渡状态小失真及H更小的距离,以晶格O原子。由于HCHO和O 2的共同吸附,在有缺陷的表面上优选使用Langmuir-Hinshelwood机理。两个C HCOH的H键解离,而被解离的H原子被吸附的O 2所吸收。因此,由于相同的氧化物质,在这两个缺陷表面上观察到了相似的活化势垒。本贡献提供了对氧化铈表面终止在HCHO氧化中的作用的分子水平的了解,从而能够进一步进行实验改进。
HCOH的H键解离,而被解离的H原子被吸附的O 2所吸收。因此,由于相同的氧化物质,在这两个缺陷表面上观察到了相似的活化势垒。本贡献提供了对氧化铈表面终止在HCHO氧化中的作用的分子水平的了解,从而能够进一步进行实验改进。



























 京公网安备 11010802027423号
京公网安备 11010802027423号