Molecular Catalysis ( IF 4.6 ) Pub Date : 2018-02-21 , DOI: 10.1016/j.mcat.2018.01.009 Anna A. Bryliakova , Andrey V. Matveev , Vladimir M. Tapilin , Vladimir V. Gorodetskii
|
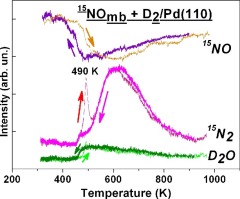
Temperature-programming desorption (TPD) and temperature-programmed reaction (TPR) have been applied to study the reduction of 15NO by deuterium on a Pd(110) surface. TPR results show that the reaction occurs in the autocatalytic regime of surface explosion with the rate-limiting step of 15NOads dissociation into highly reactive Oads and 15Nads atoms. The steady-state reaction leads to formation of 15N2, D2O, 15ND3 and 15N2O products. The phenomena of a reaction rate hysteresis observed during a heating-cooling cycle can be attributed to accumulation of 15NOads at low temperatures followed by surface explosion at T ∼ 490 K. The binding energies and structural parameters of species involved in the NO + H2 reaction over Pd(110) have been calculated by the DFT technique, and plausible reaction pathways have been considered. NO dissociation from the most stable short bridge site (Eb = −1.94 eV) occurs via the intermediates in on-top and long bridge modes with lower binding energy (Eb = −1.31 to 1.65 eV).
The energy of transition states reaches 0.2–0.26 eV over energy of NO in a gas phase, which confirms the rate-limiting role of NO dissociation. It has been demonstrated that OHads-group formation is the rate-limiting step of water molecule generation. Subsequent H2O formation occurs via disproportionation of the OHads intermediates.
中文翻译:

Pd(110)上的NO + H 2反应:TPD,TPR和DFT研究
程序升温脱附(TPD)和程序升温反应(TPR)已用于研究氘在Pd(110)表面上对15 NO的还原作用。TPR结果表明,发生在表面与爆炸的限速步骤的自催化体系的反应15 NO广告解离成反应性高的ö广告和15个Ñ广告原子。稳态反应导致形成15 N 2,D 2 O,15 ND 3和15 N 2O产品。一个加热-冷却循环过程中观察到反应速率的滞后现象可以归因于的累积15个NO广告在低温下,然后在T〜490 K的结合能和参与NO + H物种的结构参数表面爆炸通过DFT技术已经计算出在Pd(110)上的2反应,并且已经考虑了合理的反应途径。从最稳定的短桥位点(E b = -1.94 eV)发生的离解是通过具有较低结合能(E b = -1.31至1.65 eV)的顶桥和长桥模式的中间体发生的。
气相中过渡态的能量超过NO的能量达到0.2-0.26 eV,这证实了NO分解的限速作用。已经证明OH广告基团的形成是水分子生成的限速步骤。随后的H 2 O形成是通过OH ads中间体的歧化而发生的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号