Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Flow dynamics control endothelial permeability in a microfluidic vessel bifurcation model.
Lab on a Chip ( IF 6.1 ) Pub Date : 2018-02-21 00:00:00 , DOI: 10.1039/c8lc00130h Ehsan Akbari 1 , Griffin B Spychalski , Kaushik K Rangharajan , Shaurya Prakash , Jonathan W Song
Lab on a Chip ( IF 6.1 ) Pub Date : 2018-02-21 00:00:00 , DOI: 10.1039/c8lc00130h Ehsan Akbari 1 , Griffin B Spychalski , Kaushik K Rangharajan , Shaurya Prakash , Jonathan W Song
Affiliation
|
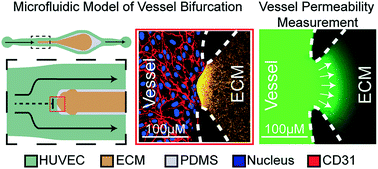
Endothelial barrier function is known to be regulated by a number of molecular mechanisms; however, the role of biomechanical signals associated with blood flow is comparatively less explored. Biomimetic microfluidic models comprised of vessel analogues that are lined with endothelial cells (ECs) have been developed to help answer several fundamental questions in endothelial mechanobiology. However, previously described microfluidic models have been primarily restricted to single straight or two parallel vessel analogues, which do not model the bifurcating vessel networks typically present in physiology. Therefore, the effects of hemodynamic stresses that arise due to bifurcating vessel geometries on ECs are not well understood. Here, we introduce and characterize a microfluidic model that mimics both the flow conditions and the endothelial/extracellular matrix (ECM) architecture of bifurcating blood vessels to systematically monitor changes in endothelial permeability mediated by the local flow dynamics at specific locations along the bifurcating vessel structure. We show that bifurcated fluid flow (BFF) that arises only at the base of a vessel bifurcation and is characterized by stagnation pressure of ∼38 dyn cm−2 and approximately zero shear stress induces significant decrease in EC permeability compared to the static control condition in a nitric oxide (NO)-dependent manner. Similarly, intravascular laminar shear stress (LSS) (3 dyn cm−2) oriented tangential to ECs located downstream of the vessel bifurcation also causes a significant decrease in permeability compared to the static control condition via the NO pathway. In contrast, co-application of transvascular flow (TVF) (∼1 μm s−1) with BFF and LSS rescues vessel permeability to the level of the static control condition, which suggests that TVF has a competing role against the stabilization effects of BFF and LSS. These findings introduce BFF at the base of vessel bifurcations as an important regulator of vessel permeability and suggest a mechanism by which local flow dynamics control vascular function in vivo.
中文翻译:

流动动力学控制微流体血管分叉模型中的内皮渗透性。
已知内皮屏障功能受多种分子机制调节。然而,与血流相关的生物力学信号的作用相对较少地被探索。已经开发了由衬有内皮细胞(EC)的血管类似物组成的仿生微流模型,以帮助回答内皮力学生物学中的几个基本问题。然而,先前描述的微流体模型主要限于单个笔直的或两个平行的血管类似物,其不对生理学中通常存在的分叉血管网络进行建模。因此,由于血管分叉的几何形状对EC产生的血流动力学应力的影响尚不清楚。这里,我们引入并表征了微流模型,该模型模仿了分叉血管的流动条件和内皮/细胞外基质(ECM)结构,以系统地监测由沿分叉血管结构的特定位置处的局部流动动力学介导的内皮通透性的变化。我们表明,分叉的流体流(BFF)仅在容器分叉的底部出现,其特征是停滞压力约为38达因厘米。与一氧化氮(NO)依赖的方式相比,与静态控制条件相比, -2和大约为零的剪切应力导致EC磁导率显着降低。类似地,与经由NO途径的静态控制条件相比,与位于血管分叉下游的EC相切的切向取向的血管内层流切应力(LSS)(3 dyn cm -2)也引起渗透率的显着降低。相反,共同应用经血管血流(TVF)(〜1μms -1)与BFF和LSS一起将血管渗透性恢复到静态控制条件的水平,这表明TVF在对抗BFF和LSS的稳定作用方面具有竞争作用。这些发现将BFF作为血管通透性的重要调节物引入了分叉血管的基础,并提出了一种通过局部流动动力学控制体内血管功能的机制。
更新日期:2018-02-21
中文翻译:

流动动力学控制微流体血管分叉模型中的内皮渗透性。
已知内皮屏障功能受多种分子机制调节。然而,与血流相关的生物力学信号的作用相对较少地被探索。已经开发了由衬有内皮细胞(EC)的血管类似物组成的仿生微流模型,以帮助回答内皮力学生物学中的几个基本问题。然而,先前描述的微流体模型主要限于单个笔直的或两个平行的血管类似物,其不对生理学中通常存在的分叉血管网络进行建模。因此,由于血管分叉的几何形状对EC产生的血流动力学应力的影响尚不清楚。这里,我们引入并表征了微流模型,该模型模仿了分叉血管的流动条件和内皮/细胞外基质(ECM)结构,以系统地监测由沿分叉血管结构的特定位置处的局部流动动力学介导的内皮通透性的变化。我们表明,分叉的流体流(BFF)仅在容器分叉的底部出现,其特征是停滞压力约为38达因厘米。与一氧化氮(NO)依赖的方式相比,与静态控制条件相比, -2和大约为零的剪切应力导致EC磁导率显着降低。类似地,与经由NO途径的静态控制条件相比,与位于血管分叉下游的EC相切的切向取向的血管内层流切应力(LSS)(3 dyn cm -2)也引起渗透率的显着降低。相反,共同应用经血管血流(TVF)(〜1μms -1)与BFF和LSS一起将血管渗透性恢复到静态控制条件的水平,这表明TVF在对抗BFF和LSS的稳定作用方面具有竞争作用。这些发现将BFF作为血管通透性的重要调节物引入了分叉血管的基础,并提出了一种通过局部流动动力学控制体内血管功能的机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号