当前位置:
X-MOL 学术
›
J. Ind. Eng. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Efficient Removal of La(III) from Water by Surface Metal Sequestration Methodology using 5-Azo-Phenolate-8-Hydroxyquinoline as a Task Designed Sequestering Material
Journal of Industrial and Engineering Chemistry ( IF 6.1 ) Pub Date : 2018-07-01 , DOI: 10.1016/j.jiec.2018.02.018 Mohamed E. Mahmoud , Asmaa K. Mohamed
Journal of Industrial and Engineering Chemistry ( IF 6.1 ) Pub Date : 2018-07-01 , DOI: 10.1016/j.jiec.2018.02.018 Mohamed E. Mahmoud , Asmaa K. Mohamed
|
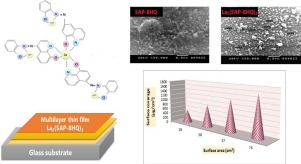
Abstract The present work explores an efficient depositive removal process of La(III) by 5-azo-phenolate-8-hydroxyquinoline (5AP-8HQ) using surface metal sequestration methodology (SMSM). Structural characterization of La(III)-complex was confirmed by different techniques. The highest surface coverage value 1597.4 μg/cm2 was recognized using pH 6.0, 0.01 mol L−1 La(III) and 76 cm2 surface area. The depositive removal of La(III) followed the Freundlich isotherm model (R2 = 0.9972) and the values of thermodynamic parameters assured spontaneous and endothermic processes. Furthermore, among the four investigated kinetics models, pseudo-second-order model (K2 = 1.15 g/mg min) yielded the best fit to describe the removal experimental data of La(III).
中文翻译:

使用 5-Azo-Phenolate-8-Hydroxyquinoline 作为任务设计的螯合材料,通过表面金属螯合方法从水中有效去除 La(III)
摘要 本工作探索了使用表面金属螯合方法 (SMSM) 通过 5-偶氮苯酚-8-羟基喹啉 (5AP-8HQ) 有效沉积去除 La(III) 的过程。La(III)-复合物的结构表征通过不同的技术得到证实。使用 pH 值 6.0、0.01 mol L-1 La(III) 和 76 cm2 表面积时识别出的最高表面覆盖值 1597.4 μg/cm2。La(III) 的沉积去除遵循 Freundlich 等温线模型 (R2 = 0.9972),热力学参数值确保自发和吸热过程。此外,在四个研究的动力学模型中,伪二级模型 (K2 = 1.15 g/mg min) 最适合描述 La(III) 的去除实验数据。
更新日期:2018-07-01
中文翻译:

使用 5-Azo-Phenolate-8-Hydroxyquinoline 作为任务设计的螯合材料,通过表面金属螯合方法从水中有效去除 La(III)
摘要 本工作探索了使用表面金属螯合方法 (SMSM) 通过 5-偶氮苯酚-8-羟基喹啉 (5AP-8HQ) 有效沉积去除 La(III) 的过程。La(III)-复合物的结构表征通过不同的技术得到证实。使用 pH 值 6.0、0.01 mol L-1 La(III) 和 76 cm2 表面积时识别出的最高表面覆盖值 1597.4 μg/cm2。La(III) 的沉积去除遵循 Freundlich 等温线模型 (R2 = 0.9972),热力学参数值确保自发和吸热过程。此外,在四个研究的动力学模型中,伪二级模型 (K2 = 1.15 g/mg min) 最适合描述 La(III) 的去除实验数据。


























 京公网安备 11010802027423号
京公网安备 11010802027423号