Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Slow‐Release Formulation of Cowpea Mosaic Virus for In Situ Vaccine Delivery to Treat Ovarian Cancer
Advanced Science ( IF 15.1 ) Pub Date : 2018-02-21 , DOI: 10.1002/advs.201700991 Anna E Czapar 1 , Brylee David B Tiu 2 , Frank A Veliz 2 , Jonathan K Pokorski 3 , Nicole F Steinmetz 2, 3, 4, 5, 6
Advanced Science ( IF 15.1 ) Pub Date : 2018-02-21 , DOI: 10.1002/advs.201700991 Anna E Czapar 1 , Brylee David B Tiu 2 , Frank A Veliz 2 , Jonathan K Pokorski 3 , Nicole F Steinmetz 2, 3, 4, 5, 6
Affiliation
|
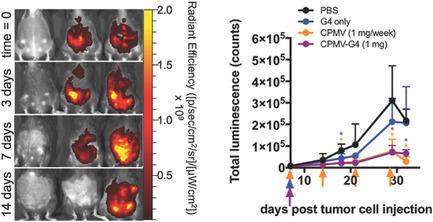
The plant viral nanoparticle cowpea mosaic virus (CPMV) is shown to be an effective immunotherapy for ovarian cancer when administered as in situ vaccine weekly, directly into the intraperitoneal (IP) space in mice with disseminated tumors. While the antitumor efficacy is promising, the required frequency of administration may pose challenges for clinical implementation. To overcome this, a slow release formulation is developed. CPMV and polyamidoamine generation 4 dendrimer form aggregates (CPMV‐G4) based on electrostatic interactions and as a function of salt concentration, allowing for tailoring of aggregate size and release of CPMV. The antitumor efficacy of a single administration of CPMV‐G4 is compared to weekly administration of soluble CPMV in a mouse model of peritoneal ovarian cancer and found to be as effective at reducing disease burden as more frequent administrations of soluble CPMV; a single injection of soluble CPMV, does not significantly slow cancer development. The ability of CPMV‐G4 to control tumor growth following a single injection is likely due to the continued presence of CPMV in the IP space leading to prolonged immune stimulation. This enhanced retention of CPMV and its antitumor efficacy demonstrates the potential for viral–dendrimer hybrids to be used for delayed release applications.
中文翻译:

用于原位疫苗递送治疗卵巢癌的豇豆花叶病毒缓释制剂
当每周一次原位疫苗直接注射到患有播散性肿瘤的小鼠的腹膜内(IP)空间时,植物病毒纳米粒子豇豆花叶病毒(CPMV)被证明是一种有效的卵巢癌免疫疗法。虽然抗肿瘤功效很有希望,但所需的给药频率可能会给临床实施带来挑战。为了克服这个问题,开发了一种缓释制剂。CPMV 和聚酰胺胺第 4 代树枝状聚合物基于静电相互作用形成聚集体 (CPMV-G4),并作为盐浓度的函数,允许定制聚集体大小和 CPMV 的释放。在腹膜卵巢癌小鼠模型中,将单次施用 CPMV-G4 与每周施用可溶性 CPMV 的抗肿瘤功效进行比较,发现在减轻疾病负担方面与更频繁施用可溶性 CPMV 一样有效;单次注射可溶性 CPMV 不会显着减缓癌症的发展。CPMV-G4 在单次注射后控制肿瘤生长的能力可能是由于 CPMV 在 IP 空间中持续存在,导致长时间的免疫刺激。CPMV 的增强保留及其抗肿瘤功效证明了病毒-树枝状大分子杂合体用于延迟释放应用的潜力。
更新日期:2018-02-21
中文翻译:

用于原位疫苗递送治疗卵巢癌的豇豆花叶病毒缓释制剂
当每周一次原位疫苗直接注射到患有播散性肿瘤的小鼠的腹膜内(IP)空间时,植物病毒纳米粒子豇豆花叶病毒(CPMV)被证明是一种有效的卵巢癌免疫疗法。虽然抗肿瘤功效很有希望,但所需的给药频率可能会给临床实施带来挑战。为了克服这个问题,开发了一种缓释制剂。CPMV 和聚酰胺胺第 4 代树枝状聚合物基于静电相互作用形成聚集体 (CPMV-G4),并作为盐浓度的函数,允许定制聚集体大小和 CPMV 的释放。在腹膜卵巢癌小鼠模型中,将单次施用 CPMV-G4 与每周施用可溶性 CPMV 的抗肿瘤功效进行比较,发现在减轻疾病负担方面与更频繁施用可溶性 CPMV 一样有效;单次注射可溶性 CPMV 不会显着减缓癌症的发展。CPMV-G4 在单次注射后控制肿瘤生长的能力可能是由于 CPMV 在 IP 空间中持续存在,导致长时间的免疫刺激。CPMV 的增强保留及其抗肿瘤功效证明了病毒-树枝状大分子杂合体用于延迟释放应用的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号