当前位置:
X-MOL 学术
›
Mol. Syst. Des. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enabling precision manufacturing of active pharmaceutical ingredients: workflow for seeded cooling continuous crystallisations†
Molecular Systems Design & Engineering ( IF 3.6 ) Pub Date : 2018-02-20 00:00:00 , DOI: 10.1039/c7me00096k Cameron J. Brown 1, 2, 3, 4 , Thomas McGlone 1, 2, 3, 4 , Stephanie Yerdelen 1, 2, 3, 4 , Vijay Srirambhatla 1, 2, 3, 4 , Fraser Mabbott 1, 2, 3, 4 , Rajesh Gurung 1, 2, 3, 4 , Maria L. Briuglia 1, 2, 3, 4 , Bilal Ahmed 1, 2, 3, 4 , Hector Polyzois 2, 3, 4, 5 , John McGinty 1, 2, 3, 4 , Francesca Perciballi 1, 2, 3, 4 , Dimitris Fysikopoulos 1, 4, 6, 7 , Pól MacFhionnghaile 1, 2, 3, 4 , Humera Siddique 1, 2, 3, 4 , Vishal Raval 1, 2, 3, 4 , Tomás S. Harrington 1, 4, 8, 9 , Antony D. Vassileiou 1, 2, 3, 4 , Murray Robertson 1, 2, 3, 4 , Elke Prasad 1, 2, 3, 4 , Andrea Johnston 1, 2, 3, 4 , Blair Johnston 1, 2, 3, 4 , Alison Nordon 1, 2, 3, 4 , Jagjit S. Srai 1, 4, 8, 9 , Gavin Halbert 1, 2, 3, 4 , Joop H. ter Horst 1, 2, 3, 4 , Chris J. Price 1, 2, 3, 4 , Chris D. Rielly 1, 4, 6, 7 , Jan Sefcik 1, 2, 3, 4 , Alastair J. Florence 1, 2, 3, 4
Molecular Systems Design & Engineering ( IF 3.6 ) Pub Date : 2018-02-20 00:00:00 , DOI: 10.1039/c7me00096k Cameron J. Brown 1, 2, 3, 4 , Thomas McGlone 1, 2, 3, 4 , Stephanie Yerdelen 1, 2, 3, 4 , Vijay Srirambhatla 1, 2, 3, 4 , Fraser Mabbott 1, 2, 3, 4 , Rajesh Gurung 1, 2, 3, 4 , Maria L. Briuglia 1, 2, 3, 4 , Bilal Ahmed 1, 2, 3, 4 , Hector Polyzois 2, 3, 4, 5 , John McGinty 1, 2, 3, 4 , Francesca Perciballi 1, 2, 3, 4 , Dimitris Fysikopoulos 1, 4, 6, 7 , Pól MacFhionnghaile 1, 2, 3, 4 , Humera Siddique 1, 2, 3, 4 , Vishal Raval 1, 2, 3, 4 , Tomás S. Harrington 1, 4, 8, 9 , Antony D. Vassileiou 1, 2, 3, 4 , Murray Robertson 1, 2, 3, 4 , Elke Prasad 1, 2, 3, 4 , Andrea Johnston 1, 2, 3, 4 , Blair Johnston 1, 2, 3, 4 , Alison Nordon 1, 2, 3, 4 , Jagjit S. Srai 1, 4, 8, 9 , Gavin Halbert 1, 2, 3, 4 , Joop H. ter Horst 1, 2, 3, 4 , Chris J. Price 1, 2, 3, 4 , Chris D. Rielly 1, 4, 6, 7 , Jan Sefcik 1, 2, 3, 4 , Alastair J. Florence 1, 2, 3, 4
Affiliation
|
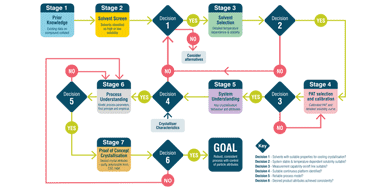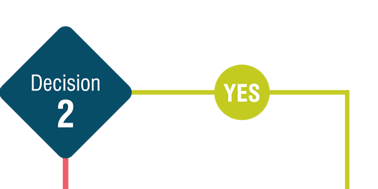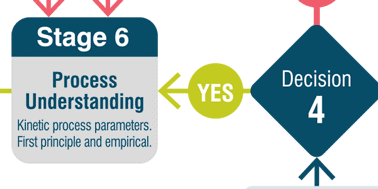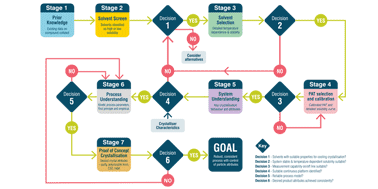
Continuous manufacturing is widely used for the production of commodity products. Currently, it is attracting increasing interest from the pharmaceutical industry and regulatory agencies as a means to provide a consistent supply of medicines. Crystallisation is a key operation in the isolation of the majority of pharmaceuticals and has been demonstrated in a continuous manner on a number of compounds using a range of processing technologies and scales. Whilst basic design principles for crystallisations and continuous processes are known, applying these in the context of rapid pharmaceutical process development with the associated constraints of speed to market and limited material availability is challenging. A systematic approach for continuous crystallisation process design is required to avoid the risk that decisions made on one aspect of the process conspire to make a later development step or steps, either for crystallisation or another unit operation, more difficult. In response to this industry challenge, an innovative system-wide approach to decision making has been developed to support rapid, systematic, and efficient continuous seeded cooling crystallisation process design. For continuous crystallisation, the goal is to develop and operate a robust, consistent process with tight control of particle attributes. Here, an innovative system-based workflow is presented that addresses this challenge. The aim, methodology, key decisions and output at each at stage are defined and a case study is presented demonstrating the successful application of the workflow for the rapid design of processes to produce kilo quantities of product with distinct, specified attributes suited to the pharmaceutical development environment. This work concludes with a vision for future applications of workflows in continuous manufacturing development to achieve rapid performance based design of pharmaceuticals.
中文翻译:

实现活性药物成分的精确制造:种子冷却连续结晶的工作流程†
连续制造被广泛用于生产商品。当前,作为提供稳定药物供应的一种手段,它吸引了制药行业和监管机构的越来越多的兴趣。结晶是分离大多数药物的关键操作,并已使用一系列加工技术和规模以连续方式在许多化合物上得到证明。尽管已知用于结晶和连续过程的基本设计原理,但是在快速制药过程开发的背景下将其应用于市场速度和有限的材料可获得性的相关限制是具有挑战性的。需要一种用于连续结晶过程设计的系统方法,以避免在该过程的一个方面做出的决定合谋使一个或多个随后的开发步骤(对于结晶或另一单元操作而言)更加困难的风险。为应对这一行业挑战,已开发出一种创新的全系统决策方法来支持快速,系统和有效的连续晶种冷却结晶工艺设计。对于连续结晶,目标是开发和运行一个鲁棒,一致的过程,并严格控制颗粒属性。在这里,提出了一个创新的基于系统的工作流程来应对这一挑战。目的,方法,定义了每个阶段的关键决策和输出,并进行了案例研究,演示了工作流程的成功应用,可快速设计流程,以生产数千吨具有独特,特定属性的产品,以适应药品开发环境。这项工作的最终目标是对工作流程在连续生产开发中的未来应用进行展望,以实现基于快速性能的药品设计。
更新日期:2018-02-20
中文翻译:

实现活性药物成分的精确制造:种子冷却连续结晶的工作流程†
连续制造被广泛用于生产商品。当前,作为提供稳定药物供应的一种手段,它吸引了制药行业和监管机构的越来越多的兴趣。结晶是分离大多数药物的关键操作,并已使用一系列加工技术和规模以连续方式在许多化合物上得到证明。尽管已知用于结晶和连续过程的基本设计原理,但是在快速制药过程开发的背景下将其应用于市场速度和有限的材料可获得性的相关限制是具有挑战性的。需要一种用于连续结晶过程设计的系统方法,以避免在该过程的一个方面做出的决定合谋使一个或多个随后的开发步骤(对于结晶或另一单元操作而言)更加困难的风险。为应对这一行业挑战,已开发出一种创新的全系统决策方法来支持快速,系统和有效的连续晶种冷却结晶工艺设计。对于连续结晶,目标是开发和运行一个鲁棒,一致的过程,并严格控制颗粒属性。在这里,提出了一个创新的基于系统的工作流程来应对这一挑战。目的,方法,定义了每个阶段的关键决策和输出,并进行了案例研究,演示了工作流程的成功应用,可快速设计流程,以生产数千吨具有独特,特定属性的产品,以适应药品开发环境。这项工作的最终目标是对工作流程在连续生产开发中的未来应用进行展望,以实现基于快速性能的药品设计。



























 京公网安备 11010802027423号
京公网安备 11010802027423号