当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Lewis Acid Catalyzed Enantioselective Desymmetrization of Donor–Acceptor meso‐Diaminocyclopropanes
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-03-23 , DOI: 10.1002/anie.201800494 Daniele Perrotta 1 , Ming-Ming Wang 1 , Jérôme Waser 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-03-23 , DOI: 10.1002/anie.201800494 Daniele Perrotta 1 , Ming-Ming Wang 1 , Jérôme Waser 1
Affiliation
|
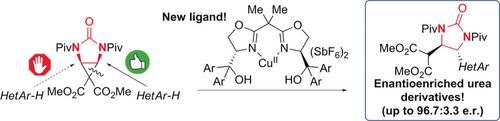
The first Lewis acid catalyzed enantioselective ring‐opening desymmetrization of a donor–acceptor meso‐diaminocyclopropane is reported. The copper(II)‐catalyzed Friedel–Crafts alkylation of indoles and one pyrrole with an unprecedented meso‐diaminocyclopropane delivered enantioenriched, diastereomerically pure urea products, which are structurally related to natural and synthetic bioactive compounds. The development of a new ligand through the investigation of an underexplored subclass of bis(oxazoline) ligands was essential for achieving high enantioselectivities.
中文翻译:

路易斯酸催化供体-受体间-二氨基环丙烷的对映选择性脱对称
据报道,路易斯酸首次催化了供体-受体内消旋二氨基环丙烷的对映选择性开环脱对称。铜(II)催化的吲哚和一个吡咯与前所未有的内消旋-二氨基环丙烷的吲哚和一个吡咯烷基化反应提供了对映体富集的,非对映体纯的尿素产品,该产品在结构上与天然和合成的生物活性化合物有关。通过研究未开发的双(恶唑啉)配体亚类,开发新的配体对于实现高对映选择性是必不可少的。
更新日期:2018-03-23
中文翻译:

路易斯酸催化供体-受体间-二氨基环丙烷的对映选择性脱对称
据报道,路易斯酸首次催化了供体-受体内消旋二氨基环丙烷的对映选择性开环脱对称。铜(II)催化的吲哚和一个吡咯与前所未有的内消旋-二氨基环丙烷的吲哚和一个吡咯烷基化反应提供了对映体富集的,非对映体纯的尿素产品,该产品在结构上与天然和合成的生物活性化合物有关。通过研究未开发的双(恶唑啉)配体亚类,开发新的配体对于实现高对映选择性是必不可少的。


























 京公网安备 11010802027423号
京公网安备 11010802027423号