当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Precursor-Receptor Interactions in the Twin Arginine Protein Transport Pathway Probed with a New Receptor Complex Preparation.
Biochemistry ( IF 2.9 ) Pub Date : 2018-02-26 , DOI: 10.1021/acs.biochem.8b00026 Marta Wojnowska 1 , Joseph Gault 2 , Shee Chien Yong 1 , Carol V Robinson 2 , Ben C Berks 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-02-26 , DOI: 10.1021/acs.biochem.8b00026 Marta Wojnowska 1 , Joseph Gault 2 , Shee Chien Yong 1 , Carol V Robinson 2 , Ben C Berks 1
Affiliation
|
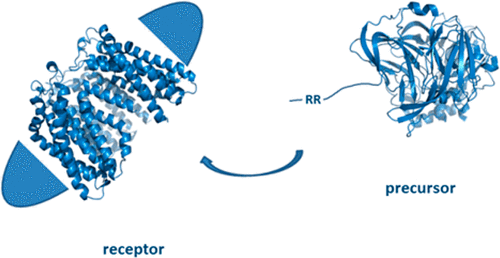
The twin arginine translocation (Tat) system moves folded proteins across the cytoplasmic membrane of bacteria and the thylakoid membrane of plant chloroplasts. Signal peptide-bearing substrates of the Tat pathway (precursor proteins) are recognized at the membrane by the TatBC receptor complex. The only established preparation of the TatBC complex uses the detergent digitonin, rendering it unsuitable for biophysical analysis. Here we show that the detergent glyco-diosgenin (GDN) can be used in place of digitonin to isolate homogeneous TatBC complexes that bind precursor proteins with physiological specificity. We use this new preparation to quantitatively characterize TatBC-precursor interactions in a fully defined system. Additionally, we show that the GDN-solubilized TatBC complex co-purifies with substantial quantities of phospholipids.
中文翻译:

前体-受体相互作用的双精氨酸蛋白运输途径探测新的受体复合物的制备。
双精氨酸易位(Tat)系统使折叠的蛋白质跨过细菌的细胞质膜和植物叶绿体的类囊体膜。TatBC受体复合物在膜上识别Tat途径的带有信号肽的底物(前体蛋白)。TatBC复合物的唯一确定的制备方法是使用洗涤剂洋地黄皂苷,使其不适用于生物物理分析。在这里,我们表明去污剂糖原-薯s皂苷元(GDN)可以代替洋地黄皂苷来分离均质的TatBC复合物,该复合物结合具有生理特异性的前体蛋白。我们使用这种新的准备工作来在完全定义的系统中定量表征TatBC-前体相互作用。此外,我们显示GDN溶解的TatBC复合物与大量磷脂共纯化。
更新日期:2018-02-20
中文翻译:

前体-受体相互作用的双精氨酸蛋白运输途径探测新的受体复合物的制备。
双精氨酸易位(Tat)系统使折叠的蛋白质跨过细菌的细胞质膜和植物叶绿体的类囊体膜。TatBC受体复合物在膜上识别Tat途径的带有信号肽的底物(前体蛋白)。TatBC复合物的唯一确定的制备方法是使用洗涤剂洋地黄皂苷,使其不适用于生物物理分析。在这里,我们表明去污剂糖原-薯s皂苷元(GDN)可以代替洋地黄皂苷来分离均质的TatBC复合物,该复合物结合具有生理特异性的前体蛋白。我们使用这种新的准备工作来在完全定义的系统中定量表征TatBC-前体相互作用。此外,我们显示GDN溶解的TatBC复合物与大量磷脂共纯化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号