当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mapping the H-NOX/HK Binding Interface in Vibrio cholerae by Hydrogen/Deuterium Exchange Mass Spectrometry
Biochemistry ( IF 2.9 ) Pub Date : 2018-02-19 00:00:00 , DOI: 10.1021/acs.biochem.8b00027 Yirui Guo , Anthony T. Iavarone , Matthew M. Cooper , Michael A. Marletta
Biochemistry ( IF 2.9 ) Pub Date : 2018-02-19 00:00:00 , DOI: 10.1021/acs.biochem.8b00027 Yirui Guo , Anthony T. Iavarone , Matthew M. Cooper , Michael A. Marletta
|
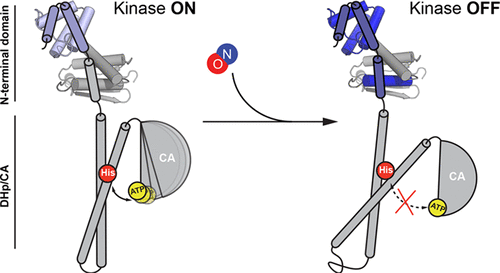
Heme-nitric oxide/oxygen binding (H-NOX) proteins are a group of hemoproteins that bind diatomic gas ligands such as nitric oxide (NO) and oxygen (O2). H-NOX proteins typically regulate histidine kinases (HK) located within the same operon. It has been reported that NO-bound H-NOXs inhibit cognate histidine kinase autophosphorylation in bacterial H-NOX/HK complexes; however, a detailed mechanism of NO-mediated regulation of the H-NOX/HK activity remains unknown. In this study, the binding interface of Vibrio cholerae (Vc) H-NOX/HK complex was characterized by hydrogen/deuterium exchange mass spectrometry (HDX-MS) and further validated by mutagenesis, leading to a new model for NO-dependent kinase inhibition. A conformational change in Vc H-NOX introduced by NO generates a new kinase-binding interface, thus locking the kinase in an inhibitory conformation.
中文翻译:

氢/氘交换质谱法绘制霍乱弧菌中H-NOX / HK结合界面的图谱
血红一氧化氮/氧结合(H-NOX)蛋白是一组结合双原子气体配体(如一氧化氮(NO)和氧(O 2))的血蛋白。H-NOX蛋白通常调节位于同一操纵子内的组氨酸激酶(HK)。据报道,NO结合的H-NOXs抑制细菌H-NOX / HK复合物中的同源组氨酸激酶自磷酸化。然而,NO介导的H-NOX / HK活性调节的详细机制仍是未知的。在这项研究中,霍乱弧菌(Vc)H-NOX / HK复合物的结合界面已通过氢/氘交换质谱(HDX-MS)进行了表征,并通过诱变得到进一步验证,从而产生了一种新的NO依赖性激酶抑制模型。Vc的构象变化 NO引入的H-NOX产生新的激酶结合界面,从而将激酶锁定在抑制性构象中。
更新日期:2018-02-19
中文翻译:

氢/氘交换质谱法绘制霍乱弧菌中H-NOX / HK结合界面的图谱
血红一氧化氮/氧结合(H-NOX)蛋白是一组结合双原子气体配体(如一氧化氮(NO)和氧(O 2))的血蛋白。H-NOX蛋白通常调节位于同一操纵子内的组氨酸激酶(HK)。据报道,NO结合的H-NOXs抑制细菌H-NOX / HK复合物中的同源组氨酸激酶自磷酸化。然而,NO介导的H-NOX / HK活性调节的详细机制仍是未知的。在这项研究中,霍乱弧菌(Vc)H-NOX / HK复合物的结合界面已通过氢/氘交换质谱(HDX-MS)进行了表征,并通过诱变得到进一步验证,从而产生了一种新的NO依赖性激酶抑制模型。Vc的构象变化 NO引入的H-NOX产生新的激酶结合界面,从而将激酶锁定在抑制性构象中。



























 京公网安备 11010802027423号
京公网安备 11010802027423号