当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Impact of Edge Groups on the Hydration and Aggregation Properties of Graphene Oxide
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2018-02-20 00:00:00 , DOI: 10.1021/acs.jpcb.8b00311 Antenor J. Paulista Neto 1 , Eudes E. Fileti 2
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2018-02-20 00:00:00 , DOI: 10.1021/acs.jpcb.8b00311 Antenor J. Paulista Neto 1 , Eudes E. Fileti 2
Affiliation
|
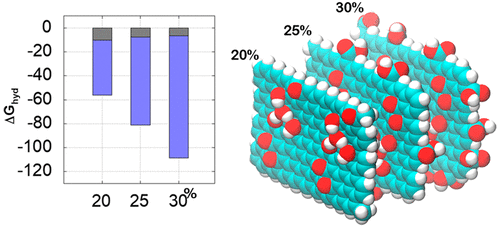
Molecular dynamics simulations were used to describe and quantify the role of edge groups on the hydrating properties of graphene oxide (GO). For this, six different oxygen concentrations were investigated, and in four of them, carboxyl groups were present. Structural analysis indicates a greater probability for the water solvation around the GO edges in detriment of the region of its basal plane, while hydrogen bonding analyses indicates that edge groups are very expressive, participating in about 60% of the total number of bonds. The impact of this bond network formed by edge groups is rationalized in energetic and thermodynamic terms. The resulting hydrophilicity observed, as expected, is of electrostatic origin and has a larger contribution from the edge groups that varies from 22 to 57% depending on the concentration. Hydration free energy and potential of mean force calculations support these findings. It was observed that the edge groups contribute up to 51% of the total hydration-free energy and that the PMF indicates the tendency for spontaneous aggregation at all investigated concentrations, being lower the higher the concentration of oxygen.
中文翻译:

边缘基团对氧化石墨烯水化和聚集性质的影响
分子动力学模拟用于描述和量化边缘基团在氧化石墨烯(GO)的水合特性中的作用。为此,研究了六种不同的氧浓度,其中有四个存在羧基。结构分析表明,GO边缘周围的水溶剂化的可能性更大,从而损害了其基面区域,而氢键分析表明,边缘基团的表达能力很强,约占键总数的60%。由边缘基团形成的这种键网络的影响在能量和热力学方面是合理的。如所预期的,所观察到的所得亲水性是静电来源的,并且根据浓度的不同,边缘基团的贡献更大,其变化范围为22%至57%。水合自由能和平均力计算的潜力支持了这些发现。观察到边缘基团贡献了高达51%的总无水合能量,并且PMF表示在所有研究的浓度下自发聚集的趋势,随着氧气浓度的升高其降低。
更新日期:2018-02-20
中文翻译:

边缘基团对氧化石墨烯水化和聚集性质的影响
分子动力学模拟用于描述和量化边缘基团在氧化石墨烯(GO)的水合特性中的作用。为此,研究了六种不同的氧浓度,其中有四个存在羧基。结构分析表明,GO边缘周围的水溶剂化的可能性更大,从而损害了其基面区域,而氢键分析表明,边缘基团的表达能力很强,约占键总数的60%。由边缘基团形成的这种键网络的影响在能量和热力学方面是合理的。如所预期的,所观察到的所得亲水性是静电来源的,并且根据浓度的不同,边缘基团的贡献更大,其变化范围为22%至57%。水合自由能和平均力计算的潜力支持了这些发现。观察到边缘基团贡献了高达51%的总无水合能量,并且PMF表示在所有研究的浓度下自发聚集的趋势,随着氧气浓度的升高其降低。



























 京公网安备 11010802027423号
京公网安备 11010802027423号