当前位置:
X-MOL 学术
›
J. Chem. Educ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dissolving Salts in Water: Students’ Particulate Explanations of Temperature Changes
Journal of Chemical Education ( IF 3 ) Pub Date : 2018-02-20 00:00:00 , DOI: 10.1021/acs.jchemed.7b00845 Timothy N. Abell 1 , Stacey Lowery Bretz 1
Journal of Chemical Education ( IF 3 ) Pub Date : 2018-02-20 00:00:00 , DOI: 10.1021/acs.jchemed.7b00845 Timothy N. Abell 1 , Stacey Lowery Bretz 1
Affiliation
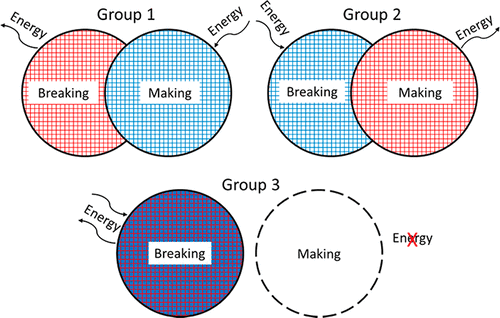
|
This study investigates how students account for a macroscopic temperature change during the dissolution of ionic salts through particulate level explanations. Semi-structured interviews were conducted with general chemistry, physical chemistry, and biophysical chemistry students. During the interviews, students conducted hands-on tasks that included the touching of beakers containing exothermic or endothermic dissolution processes. Data analysis resulted in categorizing students into groups based on their ideas about bond breaking, bond making, and energy changes. Students’ particulate understandings of the dissolving process did not appear to impact their explanations of the energy changes they observed. Only two students (one from general chemistry and one from biophysical chemistry) correctly described both the dissolving process and the macroscopic energy changes. No students invoked the concepts of potential energy, lattice energy, or enthalpy of hydration to explain their observations.
中文翻译:

在水中溶解盐:学生对温度变化的具体解释
这项研究通过颗粒水平的解释,研究了学生在离子盐溶解过程中如何解释宏观温度变化。对普通化学,物理化学和生物物理化学专业的学生进行了半结构式访谈。在面试中,学生进行了动手操作,包括触摸装有放热或吸热溶解过程的烧杯。数据分析可以根据学生对债券断裂,债券制造和能源变化的想法将他们分为几类。学生对溶解过程的颗粒理解似乎不会影响他们对所观察到的能量变化的解释。只有两名学生(一名来自普通化学,一名来自生物物理化学)正确地描述了溶解过程和宏观能量变化。没有学生会引用势能,晶格能或水合焓的概念来解释他们的观察结果。
更新日期:2018-02-20
中文翻译:

在水中溶解盐:学生对温度变化的具体解释
这项研究通过颗粒水平的解释,研究了学生在离子盐溶解过程中如何解释宏观温度变化。对普通化学,物理化学和生物物理化学专业的学生进行了半结构式访谈。在面试中,学生进行了动手操作,包括触摸装有放热或吸热溶解过程的烧杯。数据分析可以根据学生对债券断裂,债券制造和能源变化的想法将他们分为几类。学生对溶解过程的颗粒理解似乎不会影响他们对所观察到的能量变化的解释。只有两名学生(一名来自普通化学,一名来自生物物理化学)正确地描述了溶解过程和宏观能量变化。没有学生会引用势能,晶格能或水合焓的概念来解释他们的观察结果。


























 京公网安备 11010802027423号
京公网安备 11010802027423号