当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Measurement and Correlation of CO2 Solubility in 1-Ethyl-3-methylimidazolium ([EMIM]) Cation-Based Ionic Liquids: [EMIM][Ac], [EMIM][Cl], and [EMIM][MeSO4]
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2018-02-19 00:00:00 , DOI: 10.1021/acs.jced.7b00532 Joon-Hyuk Yim 1 , Seung-Jae Ha 2 , Jong Sung Lim 2
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2018-02-19 00:00:00 , DOI: 10.1021/acs.jced.7b00532 Joon-Hyuk Yim 1 , Seung-Jae Ha 2 , Jong Sung Lim 2
Affiliation
|
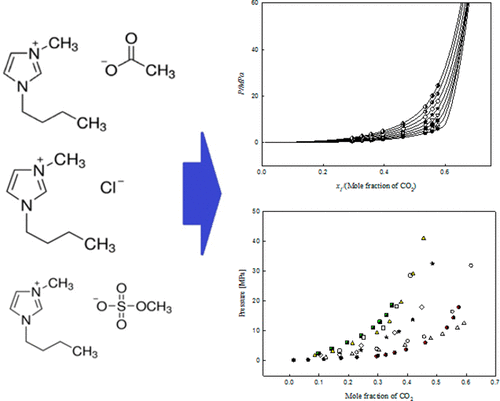
The solubility of carbon dioxide (CO2) was investigated using three different 1-ethyl-3-methylimidazolium ([EMIM]) cation-based ionic liquids: 1-ethyl-3-methylimidazolium acetate ([EMIM][Ac]), 1-ethyl-3-methylimidazolium chloride ([EMIM][Cl]), and 1-ethyl-3-methylimidazolium methyl sulfate ([EMIM][MeSO4]). The CO2 solubility data for the three ionic liquids were produced by measuring the bubble-point and cloud-point pressures of the CO2 + ionic liquid mixtures. The temperature range was from 303.15 to 403.15 K, and the pressure range was from 0.45 to 48.6 MPa. The experimental data provided results showing that the solubility of CO2 in ionic liquids increased with pressure, decreased with temperature, and was also affected by the different anions used in the experiment. The solubility is determined by CO2 mole fraction in ionic liquids, and the order of magnitude of the CO2 solubility was found to be [EMIM][Ac] > [EMIM][MeSO4] > [EMIM][Cl]. For the correlation and calculation of the experimental data, we used the Peng–Robinson equation of state, the conventional van der Waals one-fluid mixing rule, and the modified Lydersen–Joback–Reid method. The average absolute deviations of pressure were 0.0231 for CO2 + [EMIM][Ac], 0.0141 for CO2 + [EMIM][Cl], and 0.0275 for CO2 + [EMIM][MeSO4] systems.
中文翻译:

1-乙基-3-甲基咪唑鎓([EMIM])阳离子型离子液体中CO 2溶解度的测量和相关性:[EMIM] [Ac],[EMIM] [Cl]和[EMIM] [MeSO 4 ]
使用三种不同的基于1-乙基-3-甲基咪唑鎓([EMIM])阳离子的离子液体研究了二氧化碳(CO 2)的溶解度:乙酸1-乙基-3-甲基咪唑鎓([EMIM] [Ac]),1 -乙基-3-甲基咪唑鎓氯化物([EMIM] [Cl])和1-乙基-3-甲基咪唑鎓硫酸甲酯([EMIM] [MeSO 4 ])。通过测量CO 2 +离子液体混合物的起泡点和浊点压力来生成三种离子液体的CO 2溶解度数据。温度范围是303.15至403.15K,压力范围是0.45至48.6MPa。实验数据提供的结果表明,CO 2的溶解度离子液体中的离子浓度随压力的增加而增加,随温度的降低而减少,并且还受到实验中使用的不同阴离子的影响。溶解度由离子液体中的CO 2摩尔分数确定,发现CO 2溶解度的数量级为[EMIM] [Ac]> [EMIM] [MeSO 4 ]> [EMIM] [Cl]。为了对实验数据进行相关性和计算,我们使用了Peng–Robinson状态方程,常规的van der Waals单流体混合规则以及改进的Lydersen-Joback-Reid方法。的压力的平均绝对偏差分别为0.0231为CO 2 + [EMIM] [AC],0.0141为CO 2 + [EMIM] [CL],和0.0275为CO 2 + [EMIM] [内消旋4]系统。
更新日期:2018-02-21
中文翻译:

1-乙基-3-甲基咪唑鎓([EMIM])阳离子型离子液体中CO 2溶解度的测量和相关性:[EMIM] [Ac],[EMIM] [Cl]和[EMIM] [MeSO 4 ]
使用三种不同的基于1-乙基-3-甲基咪唑鎓([EMIM])阳离子的离子液体研究了二氧化碳(CO 2)的溶解度:乙酸1-乙基-3-甲基咪唑鎓([EMIM] [Ac]),1 -乙基-3-甲基咪唑鎓氯化物([EMIM] [Cl])和1-乙基-3-甲基咪唑鎓硫酸甲酯([EMIM] [MeSO 4 ])。通过测量CO 2 +离子液体混合物的起泡点和浊点压力来生成三种离子液体的CO 2溶解度数据。温度范围是303.15至403.15K,压力范围是0.45至48.6MPa。实验数据提供的结果表明,CO 2的溶解度离子液体中的离子浓度随压力的增加而增加,随温度的降低而减少,并且还受到实验中使用的不同阴离子的影响。溶解度由离子液体中的CO 2摩尔分数确定,发现CO 2溶解度的数量级为[EMIM] [Ac]> [EMIM] [MeSO 4 ]> [EMIM] [Cl]。为了对实验数据进行相关性和计算,我们使用了Peng–Robinson状态方程,常规的van der Waals单流体混合规则以及改进的Lydersen-Joback-Reid方法。的压力的平均绝对偏差分别为0.0231为CO 2 + [EMIM] [AC],0.0141为CO 2 + [EMIM] [CL],和0.0275为CO 2 + [EMIM] [内消旋4]系统。

























 京公网安备 11010802027423号
京公网安备 11010802027423号