Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2018-02-20 , DOI: 10.1016/j.bmc.2018.02.026 Hamish S Sutherland 1 , Amy S T Tong 1 , Peter J Choi 1 , Daniel Conole 1 , Adrian Blaser 1 , Scott G Franzblau 2 , Christopher B Cooper 3 , Anna M Upton 3 , Manisha U Lotlikar 3 , William A Denny 4 , Brian D Palmer 4
|
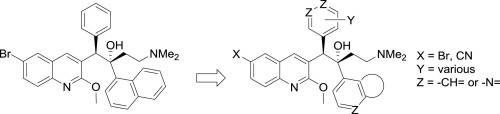
Replacing the naphthalene C-unit of the anti-tuberculosis drug bedaquiline with a range of bicyclic heterocycles of widely differing lipophilicity gave analogs with a 4.5-fold range in clogP values. The biological results for these compounds indicate on average a lower clogP limit of about 5.0 in this series for retention of potent inhibitory activity (MIC90s) against M.tb in culture. Some of the compounds also showed a significant reduction in inhibition of hERG channel potassium current compared with bedaquiline, but there was no common structural feature that distinguished these.
中文翻译:

结核病药物贝达喹啉类似物的构效关系,其中萘单元被双环杂环取代。
用一系列亲脂性差异很大的双环杂环取代抗结核药物贝达喹啉的萘 C 单元,得到 clogP 值范围为 4.5 倍的类似物。这些化合物的生物学结果表明,该系列中的 clogP 下限平均约为 5.0,可保留针对培养物中M.tb的有效抑制活性(MIC 90 s)。与贝达喹啉相比,一些化合物还显示出对 hERG 通道钾电流的抑制显着降低,但没有共同的结构特征来区分这些化合物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号