当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and characterization of thallium–salen derivatives for use as underground fluid flow tracers†
Dalton Transactions ( IF 4 ) Pub Date : 2018-02-19 00:00:00 , DOI: 10.1039/c7dt04121g Timothy J. Boyle 1, 2, 3, 4, 5 , Diana Perales 1, 2, 3, 4, 5 , Jessica M. Rimsza 1, 4, 5, 6 , Todd M. Alam 1, 4, 5, 7 , Daniel M. Boye 5, 8, 9, 10 , Jeremiah M. Sears 1, 2, 3, 4, 5 , Jeffery A. Greathouse 1, 4, 5, 6 , Richard A. Kemp 1, 2, 3, 4, 5
Dalton Transactions ( IF 4 ) Pub Date : 2018-02-19 00:00:00 , DOI: 10.1039/c7dt04121g Timothy J. Boyle 1, 2, 3, 4, 5 , Diana Perales 1, 2, 3, 4, 5 , Jessica M. Rimsza 1, 4, 5, 6 , Todd M. Alam 1, 4, 5, 7 , Daniel M. Boye 5, 8, 9, 10 , Jeremiah M. Sears 1, 2, 3, 4, 5 , Jeffery A. Greathouse 1, 4, 5, 6 , Richard A. Kemp 1, 2, 3, 4, 5
Affiliation
|
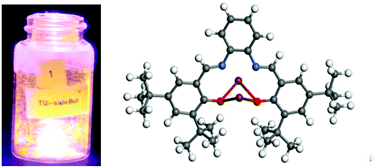
A pair of thallium salen derivatives was synthesized and characterized for potential use as monitors (or taggants) or as models for Group 13 complexes for subterranean fluid flows. These precursors were isolated from the reaction of thallium ethoxide with N,N′-bis(3,5-di-tert-butylsalicylidene)-ethylenediamine (H2-salo-But), or N,N′-bis(3,5-di-tert-butylsalicylidene)-1,2-phenylenediamine (H2-saloPh-But). The products were identified by single crystal X-ray diffraction as: [((μ-O)2,κ1-(N)(N′)salo-But)Tl2] (1) and {[((μ-O)2saloPh-But)Tl2][((μ-O)2,κ1-(N)(N′)saloPh-But)Tl2]} (2). Both structures are similar, wherein each O atom of the salo moiety bridges the two Tl atoms, leading to a Tl⋯Tl interaction, which is further stabilized by an intramolecular π-bond with neighboring phenyl rings. For 1, an additional Tl⋯N interaction was solved for each metal center; whereas, for 2, one of the two molecules in the matrix has a weak Tl⋯N interaction but no bonding noted in the other molecule. Both Density Functional Theory (DFT) calculations and variable temperature solution 205Tl NMR studies of 1 and 2 further confirmed the Tl⋯Tl interaction. The UV-vis absorbance spectra of these compounds had an absorbance peak at 392 nm for 1 and a broad absorbance peak centered at 469 nm for 2, which were found to be in good agreement with the DFT calculated spectra that were dominated by the singlet state. Fluorescence emission and excitation studies reveal absorptions at 360 and 380 nm for 1 and 2, respectively, which are attributed to the Tl⋯Tl metal centers. To demonstrate practicality, fluorescence spectra of 1 and 2 were obtained using a handheld 405 nm cw laser pointer and portable spectrometer where compound 1 was found to glow 15 times brighter than compound 2. Only compound 1 was found to survive the simulated deep-well conditions explored, which was attributed to the Tl⋯N interaction noted for 1 but not for 2.
中文翻译:

sa-salen衍生物的合成和表征,用作地下流体示踪剂†
合成了一对sale萨伦衍生物,并表征了其潜在用途,可作为监测器(或标记剂)或作为地下流体流动的第13组配合物的模型。这些前体从乙醇铊的与反应分离Ñ,Ñ ' -双(3,5-二-叔-butylsalicylidene) -乙二胺(H 2 -salo卜吨),或Ñ,ñ ' -双(3, 5-二叔丁基水杨基亚胺基)-1,2-苯二胺(H 2 -saloPh-Bu t)。产物通过单晶X射线衍射鉴定为:[((μ-O)2,κ 1 - (N)(N')萨罗卜吨)铊2 ]( 1)和{[((μ-O) 2 saloPh卜吨)铊2 ] [((μ-O) 2,κ 1 - (N)(N')saloPh卜吨)铊2 ] }( 2)。两种结构是相似的,其中sallo部分的每个O原子桥接两个T1原子,导致T1 = T1相互作用,该相互作用通过与相邻苯环的分子内π键进一步稳定。对于1,每个金属中心的Tl⋯N相互作用都得到了解决;而对于2,基质中的两个分子之一具有弱的T1N相互作用,但在另一个分子中没有发现键。1和2的密度泛函理论(DFT)计算和变温溶液205 T1 NMR研究都进一步证实了T1⋯T1相互作用。紫外可见这些化合物的吸收光谱具有在392纳米的吸收峰为1,并在469纳米的宽吸收峰值为中心为2,这被发现是在与该是由单重态为主的DFT计算光谱基本一致。荧光发射和激发研究表明1和2在360和380 nm处有吸收分别归因于Tl⋯Tl金属中心。为了证明实用性,使用手持式405 nm cw激光指示器和便携式光谱仪获得了1和2的荧光光谱,其中化合物1的发光亮度是化合物2的15倍。仅发现化合物1在探索的模拟深井条件下幸存,这归因于Tl⋯N相互作用注明为1而非2。
更新日期:2018-02-19
中文翻译:

sa-salen衍生物的合成和表征,用作地下流体示踪剂†
合成了一对sale萨伦衍生物,并表征了其潜在用途,可作为监测器(或标记剂)或作为地下流体流动的第13组配合物的模型。这些前体从乙醇铊的与反应分离Ñ,Ñ ' -双(3,5-二-叔-butylsalicylidene) -乙二胺(H 2 -salo卜吨),或Ñ,ñ ' -双(3, 5-二叔丁基水杨基亚胺基)-1,2-苯二胺(H 2 -saloPh-Bu t)。产物通过单晶X射线衍射鉴定为:[((μ-O)2,κ 1 - (N)(N')萨罗卜吨)铊2 ]( 1)和{[((μ-O) 2 saloPh卜吨)铊2 ] [((μ-O) 2,κ 1 - (N)(N')saloPh卜吨)铊2 ] }( 2)。两种结构是相似的,其中sallo部分的每个O原子桥接两个T1原子,导致T1 = T1相互作用,该相互作用通过与相邻苯环的分子内π键进一步稳定。对于1,每个金属中心的Tl⋯N相互作用都得到了解决;而对于2,基质中的两个分子之一具有弱的T1N相互作用,但在另一个分子中没有发现键。1和2的密度泛函理论(DFT)计算和变温溶液205 T1 NMR研究都进一步证实了T1⋯T1相互作用。紫外可见这些化合物的吸收光谱具有在392纳米的吸收峰为1,并在469纳米的宽吸收峰值为中心为2,这被发现是在与该是由单重态为主的DFT计算光谱基本一致。荧光发射和激发研究表明1和2在360和380 nm处有吸收分别归因于Tl⋯Tl金属中心。为了证明实用性,使用手持式405 nm cw激光指示器和便携式光谱仪获得了1和2的荧光光谱,其中化合物1的发光亮度是化合物2的15倍。仅发现化合物1在探索的模拟深井条件下幸存,这归因于Tl⋯N相互作用注明为1而非2。



























 京公网安备 11010802027423号
京公网安备 11010802027423号