当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Phosphate–phosphate oligomerization drives higher order co-assemblies with stacks of cyanostar macrocycles†
Chemical Science ( IF 8.4 ) Pub Date : 2018-02-20 00:00:00 , DOI: 10.1039/c7sc05290a Elisabeth M. Fatila 1, 2, 3, 4 , Maren Pink 1, 2, 3, 4 , Eric B. Twum 1, 2, 3, 4 , Jonathan A. Karty 1, 2, 3, 4 , Amar H. Flood 1, 2, 3, 4
Chemical Science ( IF 8.4 ) Pub Date : 2018-02-20 00:00:00 , DOI: 10.1039/c7sc05290a Elisabeth M. Fatila 1, 2, 3, 4 , Maren Pink 1, 2, 3, 4 , Eric B. Twum 1, 2, 3, 4 , Jonathan A. Karty 1, 2, 3, 4 , Amar H. Flood 1, 2, 3, 4
Affiliation
|
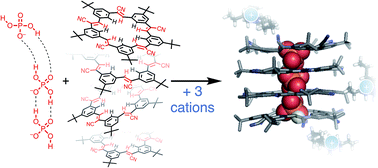
The importance of phosphate in biology and chemistry has long motivated investigation of its recognition. Despite this interest, phosphate's facile oligomerization is only now being examined following the discovery of complexes of anion–anion dimers of hydroxyanions. Here we address how oligomerization dictates phosphate's recognition properties when engaged with planar cyanostar macrocycles that can also oligomerize by stacking. The crystal structure of cyanostar with phosphate shows an unprecedented tetrameric stack of cyanostar macrocycles threaded by a phosphate trimer, [H2PO4⋯H2PO4⋯H2PO4]3−. The solution behaviour, studied as a function of solvent quality, highlights how dimers and trimers of phosphate drive formation of higher order stacks of cyanostar into dimer, trimer and tetramer co-assemblies. Solution behaviors differ significantly from simpler complexes of bisulfate hydroxyanion dimers. Phosphate oligomerization is: (1) preferred over ion pairing with tetrabutylammonium cations, (2) inhibits disassembly of the complexes upon dilution, and (3) resists interference from competitive anion solvation. The phosphate oligomers also appear critical for stability; complexation of just one phosphate with cyanostars is unfavored. The cyanostar's ability to self-assemble is found to create a tubular, highly electropositive cavity that complements the size and shape of the phosphate oligomers as well as their higher charge. When given the opportunity, phosphate will cooperate with the receptor to form co-assembled architectures.
中文翻译:

磷酸盐-磷酸盐的低聚反应可与氰基星形大环化合物的堆积推动更高阶的共组装体†
磷酸盐在生物学和化学中的重要性长期以来一直引起人们对其认可的研究。尽管有这种兴趣,但在发现阴离子-羟基阴离子的阴离子二聚体的配合物之后,才对磷酸盐的轻度低聚进行研究。在这里,我们讨论低聚化如何与平面氰基大环(也可以通过堆叠低聚)结合时决定磷酸酯的识别特性。带有磷酸盐的氰基星形晶体的晶体结构显示出前所未有的四聚体状氰基星形大环,其被磷酸盐三聚体,[H 2 PO 4 ⋯H 2 PO 4 ⋯H 2 PO 4 ] 3−穿线。。作为溶剂质量的函数而研究的溶液行为突出显示了磷酸盐的二聚体和三聚体如何驱动氰化星高阶堆栈的形成为二聚体,三聚体和四聚体组合。溶液的行为与硫酸氢根羟基阴离子二聚体的简单络合物有很大不同。磷酸盐低聚是:(1)优于与四丁基铵阳离子的离子对,(2)抑制稀释时络合物的分解,和(3)抵抗竞争性阴离子溶剂化的干扰。磷酸盐低聚物对稳定性也很关键。仅将一种磷酸盐与氰基明星络合是不利的。发现cyanostar的自组装能力可形成管状的高电正性腔,该腔可补充磷酸盐低聚物的大小和形状以及其较高的电荷。
更新日期:2018-02-20
中文翻译:

磷酸盐-磷酸盐的低聚反应可与氰基星形大环化合物的堆积推动更高阶的共组装体†
磷酸盐在生物学和化学中的重要性长期以来一直引起人们对其认可的研究。尽管有这种兴趣,但在发现阴离子-羟基阴离子的阴离子二聚体的配合物之后,才对磷酸盐的轻度低聚进行研究。在这里,我们讨论低聚化如何与平面氰基大环(也可以通过堆叠低聚)结合时决定磷酸酯的识别特性。带有磷酸盐的氰基星形晶体的晶体结构显示出前所未有的四聚体状氰基星形大环,其被磷酸盐三聚体,[H 2 PO 4 ⋯H 2 PO 4 ⋯H 2 PO 4 ] 3−穿线。。作为溶剂质量的函数而研究的溶液行为突出显示了磷酸盐的二聚体和三聚体如何驱动氰化星高阶堆栈的形成为二聚体,三聚体和四聚体组合。溶液的行为与硫酸氢根羟基阴离子二聚体的简单络合物有很大不同。磷酸盐低聚是:(1)优于与四丁基铵阳离子的离子对,(2)抑制稀释时络合物的分解,和(3)抵抗竞争性阴离子溶剂化的干扰。磷酸盐低聚物对稳定性也很关键。仅将一种磷酸盐与氰基明星络合是不利的。发现cyanostar的自组装能力可形成管状的高电正性腔,该腔可补充磷酸盐低聚物的大小和形状以及其较高的电荷。



























 京公网安备 11010802027423号
京公网安备 11010802027423号