Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2018-02-16 , DOI: 10.1016/j.bmc.2018.02.024 Xin Wei , Zhi Dai , Jing Yang , Afsar Khan , Hao-Fei Yu , Yun-Li Zhao , Yi-Fen Wang , Ya-Ping Liu , Zi-Feng Yang , Wan-Yi Huang , Xin-Hua Wang , Xu-Dong Zhao , Xiao-Dong Luo
|
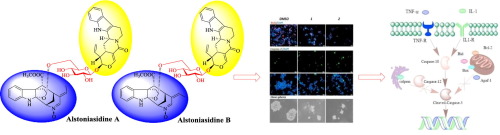
Unlike reported bisindoles linked by single bond directly, alstoniasidines A (1) and B (2), from Alstonia scholaris featuring unprecedented skeleton with two indole moieties bridged by a sugar, represented a novel bisindole type having strictosamide-glucopyranose-picraline scaffold. Both compounds exhibited selective cytotoxicity against human glioma stem cells (GSCs) and induced caspase-3 dependent extrinsic apoptosis by increasing the expression of interleukin 1 (IL-1), tumor necrosis factor (TNF-α), and the cleaved caspase-3, while damaged the unlimited proliferation and self-renewal capacity of GSCs. This finding might provide new type of leads for the selective killing of human glioma stem cells.
中文翻译:

前所未有的糖桥双吲哚选择性抑制神经胶质瘤干细胞
与报道的通过单键直接连接的双吲哚不同,来自Alstonia Scholaris的alstoniasidines A(1)和B(2)具有前所未有的骨架,带有两个由糖桥接的吲哚部分,代表了一种新型双吲哚类型,其具有严格的酰胺-吡喃葡萄糖-吡啶骨架。两种化合物均通过增加白介素1(IL-1),肿瘤坏死因子(TNF-α)和裂解的caspase-3的表达,表现出对人神经胶质瘤干细胞(GSCs)的选择性细胞毒性,并诱导caspase-3依赖性外源性凋亡,同时破坏了GSC的无限扩散和自我更新能力。这一发现可能为选择性杀死人神经胶质瘤干细胞提供新型线索。


























 京公网安备 11010802027423号
京公网安备 11010802027423号