当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Two Stereoinduction Events in One C−H Activation Step: A Route towards Terphenyl Ligands with Two Atropisomeric Axes
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-03-13 , DOI: 10.1002/anie.201801130 Quentin Dherbassy 1 , Jean-Pierre Djukic 2 , Joanna Wencel-Delord 1 , Françoise Colobert 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-03-13 , DOI: 10.1002/anie.201801130 Quentin Dherbassy 1 , Jean-Pierre Djukic 2 , Joanna Wencel-Delord 1 , Françoise Colobert 1
Affiliation
|
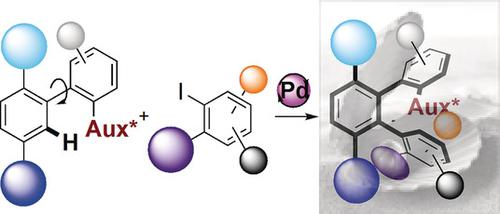
Herein we disclose the synthesis of original chiral scaffolds—ortho‐orientated terphenyls presenting two atropisomeric Ar–Ar axes. These unusual structures were built up by using the C−H activation approach, and remarkably, both chiral axes were controlled with excellent stereoselectivity in a single transformation. During the reaction, not only does atroposelective functionalization of a biaryl precursor occur to establish one stereogenic axis, but an unprecedented atropo‐stereoselective C−H arylation also takes place to generate the second stereogenic element. These enantiomerically pure ortho‐terphenyls show an original tridimensional structure and thus constitute a unique foundation for building up a library of enantiomerically pure bidentate ligands, such as the new ligands S/N‐Biax and diphosphine BiaxPhos.
中文翻译:

在一个CH活化步骤中发生两个立体诱导事件:通往带有两个阻转异构体轴的三联苯配体的途径
在这里,我们公开了原始手性支架的合成-呈现两个阻转异构Ar-Ar轴的邻位三联苯。这些不寻常的结构是通过使用CH活化方法建立的,并且值得注意的是,两个手性轴在一次转化中都具有出色的立体选择性。在反应过程中,不仅会发生联芳基前体的对映选择性官能化以建立一个立体轴,而且还会发生空前的对映立体选择性CH芳基化反应,从而生成第二个立体成因元素。这些对映体纯的邻位叔苯基具有原始的三维结构,因此为建立对映体纯的双齿配体(例如新的配体S / N-Biax和二膦酸BiaxPhos)库提供了独特的基础。
更新日期:2018-03-13
中文翻译:

在一个CH活化步骤中发生两个立体诱导事件:通往带有两个阻转异构体轴的三联苯配体的途径
在这里,我们公开了原始手性支架的合成-呈现两个阻转异构Ar-Ar轴的邻位三联苯。这些不寻常的结构是通过使用CH活化方法建立的,并且值得注意的是,两个手性轴在一次转化中都具有出色的立体选择性。在反应过程中,不仅会发生联芳基前体的对映选择性官能化以建立一个立体轴,而且还会发生空前的对映立体选择性CH芳基化反应,从而生成第二个立体成因元素。这些对映体纯的邻位叔苯基具有原始的三维结构,因此为建立对映体纯的双齿配体(例如新的配体S / N-Biax和二膦酸BiaxPhos)库提供了独特的基础。



























 京公网安备 11010802027423号
京公网安备 11010802027423号