当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development of a Focused Library of Triazole‐Linked Privileged‐Structure‐Based Conjugates Leading to the Discovery of Novel Phenotypic Hits against Protozoan Parasitic Infections
ChemMedChem ( IF 3.4 ) Pub Date : 2018-02-16 , DOI: 10.1002/cmdc.201700786 Elisa Uliassi 1 , Lorna Piazzi 1 , Federica Belluti 1 , Andrea Mazzanti 2 , Marcel Kaiser 3, 4 , Reto Brun 3, 4 , Carolina B. Moraes 5, 6 , Lucio H. Freitas-Junior 5, 6 , Sheraz Gul 7 , Maria Kuzikov 7 , Bernhard Ellinger 7 , Chiara Borsari 8 , Maria Paola Costi 8 , Maria Laura Bolognesi 1
ChemMedChem ( IF 3.4 ) Pub Date : 2018-02-16 , DOI: 10.1002/cmdc.201700786 Elisa Uliassi 1 , Lorna Piazzi 1 , Federica Belluti 1 , Andrea Mazzanti 2 , Marcel Kaiser 3, 4 , Reto Brun 3, 4 , Carolina B. Moraes 5, 6 , Lucio H. Freitas-Junior 5, 6 , Sheraz Gul 7 , Maria Kuzikov 7 , Bernhard Ellinger 7 , Chiara Borsari 8 , Maria Paola Costi 8 , Maria Laura Bolognesi 1
Affiliation
|
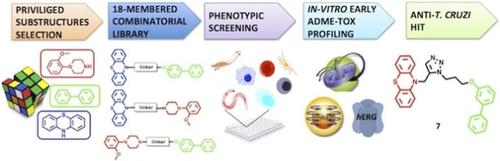
Protozoan infections caused by Plasmodium, Leishmania, and Trypanosoma spp. contribute significantly to the burden of infectious diseases worldwide, causing severe morbidity and mortality. The inadequacy of available treatments calls for cost‐ and time‐effective drug discovery endeavors. To this end, we envisaged the triazole linkage of privileged structures as an effective drug design strategy to generate a focused library of high‐quality compounds. The versatility of this approach was combined with the feasibility of a phenotypic assay, integrated with early ADME‐tox profiling. Thus, an 18‐membered library was efficiently assembled via Huisgen cycloaddition of phenothiazine, biphenyl, and phenylpiperazine scaffolds. The resulting 18 compounds were then tested against seven parasite strains, and counter‐screened for selectivity against two mammalian cell lines. In parallel, hERG and cytochrome P450 (CYP) inhibition, and mitochondrial toxicity were assessed. Remarkably, 10‐((1‐(3‐([1,1′‐biphenyl]‐3‐yloxy)propyl)‐1H‐1,2,3‐triazol‐5‐yl)methyl)‐10H‐phenothiazine (7) and 10‐(3‐(1‐(3‐([1,1′‐biphenyl]‐3‐yloxy)propyl)‐1H‐1,2,3‐triazol‐4‐yl)propyl)‐10H‐phenothiazine (12) showed respective IC50 values of 1.8 and 1.9 μg mL−1 against T. cruzi, together with optimal selectivity. In particular, compound 7 showed a promising ADME‐tox profile. Thus, hit 7 might be progressed as an antichagasic lead.
中文翻译:

基于三唑连接的特权结构的缀合物的重点文库的开发,导致发现了针对原生动物寄生虫感染的新型表型
疟原虫,利什曼原虫和锥虫引起的原生动物感染spp。对全世界的传染病负担做出重大贡献,导致严重的发病率和死亡率。现有治疗方法的不足要求进行具有成本效益和时间效益的药物开发。为此,我们设想将特权结构的三唑键连接作为有效的药物设计策略,以生成高质量化合物的重点文库。这种方法的多功能性与表型分析的可行性相结合,并与早期的ADME-tox分析相结合。因此,通过吩噻嗪,联苯和苯基哌嗪支架的Huisgen环加成反应,可以有效地组装一个由18个成员组成的文库。然后对所得的18种化合物针对7种寄生虫菌株进行了测试,并针对两种哺乳动物细胞系进行了反筛选。平行地,h评估了ERG和细胞色素P450(CYP)的抑制作用以及线粒体毒性。值得注意的是,10-((1-(3-([[1,1'-联苯] -3-基氧基)丙基)-1 H -1,1,2,3-三唑-5-基)甲基)-10 H-吩噻嗪(7)和10-(3-(1-(3-([[1,1'-联苯] -3-基氧基)丙基)-1 H -1,2,3-三唑-4-基)丙基)- 10 H-吩噻嗪(12)分别显示了针对克氏锥虫的IC 50值为1.8和1.9μgmL -1,同时具有最佳的选择性。尤其是,化合物7显示出有希望的ADME毒素谱。因此,命中7可能会成为反主角的线索。
更新日期:2018-02-16
中文翻译:

基于三唑连接的特权结构的缀合物的重点文库的开发,导致发现了针对原生动物寄生虫感染的新型表型
疟原虫,利什曼原虫和锥虫引起的原生动物感染spp。对全世界的传染病负担做出重大贡献,导致严重的发病率和死亡率。现有治疗方法的不足要求进行具有成本效益和时间效益的药物开发。为此,我们设想将特权结构的三唑键连接作为有效的药物设计策略,以生成高质量化合物的重点文库。这种方法的多功能性与表型分析的可行性相结合,并与早期的ADME-tox分析相结合。因此,通过吩噻嗪,联苯和苯基哌嗪支架的Huisgen环加成反应,可以有效地组装一个由18个成员组成的文库。然后对所得的18种化合物针对7种寄生虫菌株进行了测试,并针对两种哺乳动物细胞系进行了反筛选。平行地,h评估了ERG和细胞色素P450(CYP)的抑制作用以及线粒体毒性。值得注意的是,10-((1-(3-([[1,1'-联苯] -3-基氧基)丙基)-1 H -1,1,2,3-三唑-5-基)甲基)-10 H-吩噻嗪(7)和10-(3-(1-(3-([[1,1'-联苯] -3-基氧基)丙基)-1 H -1,2,3-三唑-4-基)丙基)- 10 H-吩噻嗪(12)分别显示了针对克氏锥虫的IC 50值为1.8和1.9μgmL -1,同时具有最佳的选择性。尤其是,化合物7显示出有希望的ADME毒素谱。因此,命中7可能会成为反主角的线索。

























 京公网安备 11010802027423号
京公网安备 11010802027423号