当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Paired electrochemical conversion of nitroarenes to sulfonamides, diarylsulfones and bis(arylsulfonyl)aminophenols
Green Chemistry ( IF 9.8 ) Pub Date : 2018-02-16 00:00:00 , DOI: 10.1039/c7gc03576d Banafsheh Mokhtari 1, 2, 3, 4 , Davood Nematollahi 1, 2, 3, 4 , Hamid Salehzadeh 1, 4, 5, 6
Green Chemistry ( IF 9.8 ) Pub Date : 2018-02-16 00:00:00 , DOI: 10.1039/c7gc03576d Banafsheh Mokhtari 1, 2, 3, 4 , Davood Nematollahi 1, 2, 3, 4 , Hamid Salehzadeh 1, 4, 5, 6
Affiliation
|
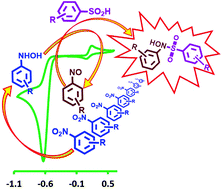
A paired electrochemical method using nitrobenzene (NB) derivatives and arylsulfinic acids (ASAs) as starting materials was developed for the synthesis of some new sulfonamides, diarylsulfones and bis(arylsulfonyl)aminophenols. The synthetic strategy was designed using the data provided by electrochemical studies involving cyclic voltammetry on NB oxidation in the absence and presence of ASAs. The reactions have been successfully performed in an undivided cell, at carbon rod electrodes, in aqueous solutions, by constant current electrolysis at room temperature. This strategy does not require catalysts, toxic solvents and challenging workups. It is also applicable for a wide range of nitroarenes.
中文翻译:

硝基芳烃成对电化学转化为磺酰胺,二芳基砜和双(芳基磺酰基)氨基酚
开发了一种以硝基苯(NB)衍生物和芳基亚磺酸(ASAs)为起始原料的成对电化学方法,用于合成一些新的磺酰胺,二芳基砜和双(芳基磺酰基)氨基酚。合成策略是根据在不存在和存在ASA的情况下,电化学研究提供的数据(涉及循环伏安法对NB氧化)设计的。通过在室温下恒流电解,该反应已成功地在不分隔的槽中,在碳棒电极上,在水溶液中进行。该策略不需要催化剂,有毒溶剂和具有挑战性的后处理。它也适用于各种硝基芳烃。
更新日期:2018-04-03
中文翻译:

硝基芳烃成对电化学转化为磺酰胺,二芳基砜和双(芳基磺酰基)氨基酚
开发了一种以硝基苯(NB)衍生物和芳基亚磺酸(ASAs)为起始原料的成对电化学方法,用于合成一些新的磺酰胺,二芳基砜和双(芳基磺酰基)氨基酚。合成策略是根据在不存在和存在ASA的情况下,电化学研究提供的数据(涉及循环伏安法对NB氧化)设计的。通过在室温下恒流电解,该反应已成功地在不分隔的槽中,在碳棒电极上,在水溶液中进行。该策略不需要催化剂,有毒溶剂和具有挑战性的后处理。它也适用于各种硝基芳烃。



























 京公网安备 11010802027423号
京公网安备 11010802027423号