当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Confinement Effects on the Benzene Orientational Structure
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-03-13 , DOI: 10.1002/anie.201713115 Marta Falkowska 1 , Daniel T. Bowron 2 , Haresh Manyar 3 , Tristan G. A. Youngs 2 , Christopher Hardacre 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-03-13 , DOI: 10.1002/anie.201713115 Marta Falkowska 1 , Daniel T. Bowron 2 , Haresh Manyar 3 , Tristan G. A. Youngs 2 , Christopher Hardacre 1
Affiliation
|
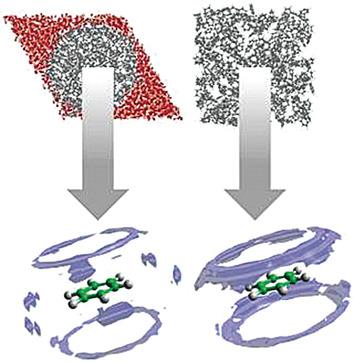
Liquids under confinement exhibit different properties compared with their corresponding bulk phases, for example, miscibility, phase transitions, and diffusion. The underlying cause is the local ordering of molecules, which is usually only studied using pure simulation methods. Herein, we derive experimentally the structure of benzene confined in MCM‐41 using total neutron scattering measurements. The study reveals a layering of molecules across a pore, and four concentric cylindrical shells can be distinguished for a pore with the radius of 18 Å. The nanoscale confinement of the liquid has a major effect on the spatial and orientational correlations observed between the molecules, when compared with the structure of the bulk liquid. These differences are most marked for molecules in parallel configurations, and this suggests differences in chemical reactivity between the confined and bulk liquids.
中文翻译:

限制对苯取向结构的影响
与相应的本体相相比,处于受限状态的液体表现出不同的性质,例如,混溶性,相变和扩散。根本原因是分子的局部排序,通常仅使用纯模拟方法进行研究。本文中,我们使用总中子散射测量值,通过实验推导了MCM-41中所含苯的结构。该研究揭示了整个孔隙中分子的分层,对于半径为18Å的孔隙,可以区分出四个同心圆柱壳。当与本体液体的结构相比时,液体的纳米级限制对观察到的分子之间的空间和取向相关性具有重大影响。这些差异在平行构型的分子中最为明显,
更新日期:2018-03-13
中文翻译:

限制对苯取向结构的影响
与相应的本体相相比,处于受限状态的液体表现出不同的性质,例如,混溶性,相变和扩散。根本原因是分子的局部排序,通常仅使用纯模拟方法进行研究。本文中,我们使用总中子散射测量值,通过实验推导了MCM-41中所含苯的结构。该研究揭示了整个孔隙中分子的分层,对于半径为18Å的孔隙,可以区分出四个同心圆柱壳。当与本体液体的结构相比时,液体的纳米级限制对观察到的分子之间的空间和取向相关性具有重大影响。这些差异在平行构型的分子中最为明显,


























 京公网安备 11010802027423号
京公网安备 11010802027423号