当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Increased Degree of Unsaturation in the Lipid of Antifungal Cationic Amphiphiles Facilitates Selective Fungal Cell Disruption
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2018-02-08 00:00:00 , DOI: 10.1021/acsinfecdis.7b00272 Kfir B. Steinbuch 1 , Raphael I. Benhamou 1 , Lotan Levin 2 , Reuven Stein 2 , Micha Fridman 1
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2018-02-08 00:00:00 , DOI: 10.1021/acsinfecdis.7b00272 Kfir B. Steinbuch 1 , Raphael I. Benhamou 1 , Lotan Levin 2 , Reuven Stein 2 , Micha Fridman 1
Affiliation
|
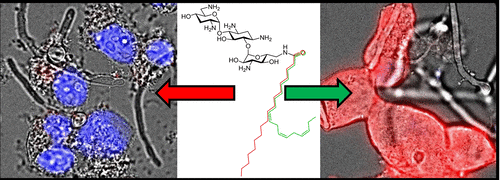
Antimicrobial cationic amphiphiles derived from aminoglycosides act through cell membrane permeabilization but have limited selectivity for microbial cell membranes. Herein, we report that an increased degree of unsaturation in the fatty acid segment of antifungal cationic amphiphiles derived from the aminoglycoside tobramycin significantly reduced toxicity to mammalian cells. A collection of tobramycin-derived cationic amphiphiles substituted with C18 lipid chains varying in degree of unsaturation and double bond configuration were synthesized. All had potent activity against a panel of important fungal pathogens including strains with resistance to a variety of antifungal drugs. The tobramycin-derived cationic amphiphile substituted with linolenic acid with three cis double bonds (compound 6) was up to an order of magnitude less toxic to mammalian cells than cationic amphiphiles composed of lipids with a lower degree of unsaturation and than the fungal membrane disrupting drug amphotericin B. Compound 6 was 12-fold more selective (red blood cell hemolysis relative to antifungal activity) than compound 1, the derivative with a fully saturated lipid chain. Notably, compound 6 disrupted the membranes of fungal cells without affecting the viability of cocultured mammalian cells. This study demonstrates that the degree of unsaturation and the configuration of the double bond in lipids of cationic amphiphiles are important parameters that, if optimized, result in compounds with broad spectrum and potent antifungal activity as well as reduced toxicity toward mammalian cells.
中文翻译:

抗真菌阳离子两亲物的脂质中不饱和度的增加促进选择性真菌细胞破裂。
衍生自氨基糖苷的抗菌阳离子两亲物通过细胞膜通透作用发挥作用,但对微生物细胞膜的选择性有限。在本文中,我们报道了衍生自氨基糖苷妥布霉素的抗真菌阳离子两亲物的脂肪酸区段中的不饱和度的增加程度显着降低了对哺乳动物细胞的毒性。合成了由妥布霉素衍生的阳离子两亲物的集合,这些阳离子两亲物被不饱和度和双键构型不同的C 18脂质链取代。它们均对一组重要的真菌病原体具有有效的活性,这些病原体包括对多种抗真菌药物具有抗性的菌株。妥布霉素衍生的阳离子两亲物被亚麻酸与三个顺式取代双键(化合物6)对哺乳动物细胞的毒性比阳离子两亲物(由不饱和度较低的脂质组成,并且比破坏真菌膜的两性霉素B的阳离子两亲物)毒性低一个数量级。化合物6的选择性高12倍(红色血细胞溶血相对于抗真菌活性而言)比化合物1的衍生物具有完全饱和的脂质链。值得注意的是,化合物6在不影响共培养哺乳动物细胞活力的情况下破坏了真菌细胞的膜。这项研究表明,阳离子两亲物的脂质中的不饱和度和双键的构型是重要的参数,如果进行了优化,它们将导致化合物具有广谱和有效的抗真菌活性,并降低对哺乳动物细胞的毒性。
更新日期:2018-02-08
中文翻译:

抗真菌阳离子两亲物的脂质中不饱和度的增加促进选择性真菌细胞破裂。
衍生自氨基糖苷的抗菌阳离子两亲物通过细胞膜通透作用发挥作用,但对微生物细胞膜的选择性有限。在本文中,我们报道了衍生自氨基糖苷妥布霉素的抗真菌阳离子两亲物的脂肪酸区段中的不饱和度的增加程度显着降低了对哺乳动物细胞的毒性。合成了由妥布霉素衍生的阳离子两亲物的集合,这些阳离子两亲物被不饱和度和双键构型不同的C 18脂质链取代。它们均对一组重要的真菌病原体具有有效的活性,这些病原体包括对多种抗真菌药物具有抗性的菌株。妥布霉素衍生的阳离子两亲物被亚麻酸与三个顺式取代双键(化合物6)对哺乳动物细胞的毒性比阳离子两亲物(由不饱和度较低的脂质组成,并且比破坏真菌膜的两性霉素B的阳离子两亲物)毒性低一个数量级。化合物6的选择性高12倍(红色血细胞溶血相对于抗真菌活性而言)比化合物1的衍生物具有完全饱和的脂质链。值得注意的是,化合物6在不影响共培养哺乳动物细胞活力的情况下破坏了真菌细胞的膜。这项研究表明,阳离子两亲物的脂质中的不饱和度和双键的构型是重要的参数,如果进行了优化,它们将导致化合物具有广谱和有效的抗真菌活性,并降低对哺乳动物细胞的毒性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号