Journal of the American Society for Mass Spectrometry ( IF 3.2 ) Pub Date : 2018-02-15 , DOI: 10.1007/s13361-018-1894-1 Bradley B. Stocks 1 , Jeremy E. Melanson 1
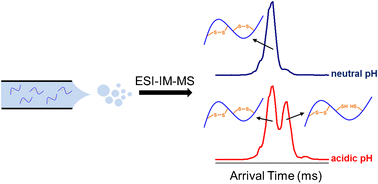
Many peptides with antimicrobial activity and/or therapeutic potential contain disulfide bonds as a means to enhance stability, and their quantitation is often performed using electrospray ionization mass spectrometry (ESI-MS). Disulfides can be reduced during ESI under commonly used instrument conditions, which has the potential to hinder accurate peptide quantitation. We demonstrate that this in-source reduction (ISR) is predominantly observed for peptides infused from acidic solutions and subjected to elevated ESI voltages (3–4 kV). ISR is readily apparent in the mass spectrum of oxytocin—a small, single disulfide-containing peptide. However, subtle m/z shifts due to partial ISR of highly charged (z ≥ 3) peptides with multiple disulfide linkages may proceed unnoticed. Ion mobility (IM)-MS separates ions on the basis of charge and shape in the gas phase, and using insulin as a model system, we show that IM-MS arrival time distributions (ATDs) are particularly sensitive to partial ISR of large peptides. Isotope modeling allows for the relative quantitation of disulfide-intact and partially reduced states of the mobility-separated peptide conformers. Interestingly, hepcidin peptides ionized from acidic solutions at elevated ESI voltages undergo gas-phase compaction, ostensibly due to partial disulfide ISR. Our IM-MS results lead us to propose that residual acid is the likely cause of disparate ATDs recently measured for hepcidin from different suppliers [Anal. Bioanal. Chem. 409, 2559–2567 (2017)]. Overall, our results demonstrate the utility of IM-MS to detect partial ISR of disulfide-bonded peptides and reinforce the notion that peptide/protein measurements should be carried out using minimally activating instrument conditions.
ᅟ
中文翻译:

离子迁移质谱法监测二硫键结合肽的源内还原
许多具有抗菌活性和/或治疗潜力的肽都包含二硫键,以增强稳定性,其定量通常使用电喷雾电离质谱(ESI-MS)进行。在ESI中,在常用的仪器条件下可以减少二硫键,这有可能阻碍准确的肽定量。我们证明,这种源内还原(ISR)主要观察到从酸性溶液注入的肽并经受较高的ESI电压(3-4 kV)。ISR在催产素(一种小的,单一的含二硫键的肽)的质谱图中显而易见。然而,细微米/ Ž偏移由于高度带电的(部分ISR ž ≥3)具有多个二硫键的肽可能会被忽视。离子淌度(IM)-MS根据气相中的电荷和形状分离离子,并使用胰岛素作为模型系统,我们证明IM-MS到达时间分布(ATDs)对大肽的部分ISR尤其敏感。同位素建模可以相对定量地定量分析迁移率分离的肽构象异构体的二硫键完整和部分还原的状态。有趣的是,表面上由于部分二硫键ISR,从酸性溶液中离子化的铁调素肽会经历气相压实。我们的IM-MS结果使我们建议,残留酸是最近从不同供应商处检测到的铁调素中不同ATD的可能原因[Anal。生物肛门。化学 409,2559–2567(2017)]。总体而言,我们的结果表明IM-MS可用于检测二硫键结合的肽的部分ISR,并强化了这样的观念,即应该使用最小程度地激活仪器条件进行肽/蛋白质测量。

ᅟ

























 京公网安备 11010802027423号
京公网安备 11010802027423号