当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tuning Inner-Ear Tip-Link Affinity Through Alternatively Spliced Variants of Protocadherin-15
Biochemistry ( IF 2.9 ) Pub Date : 2018-02-14 00:00:00 , DOI: 10.1021/acs.biochem.7b01075 Yoshie Narui 1 , Marcos Sotomayor 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-02-14 00:00:00 , DOI: 10.1021/acs.biochem.7b01075 Yoshie Narui 1 , Marcos Sotomayor 1
Affiliation
|
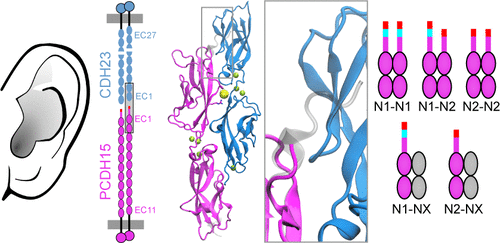
Human hearing relies upon the tip-to-tip interaction of two nonclassical cadherins, protocadherin-15 (PCDH15) and cadherin-23 (CDH23). Together, these proteins form a filament called the tip link that connects neighboring stereocilia of mechanosensitive hair cells. As sound waves enter the cochlea, the stereocilia deflect and tension is applied to the tip link, opening nearby transduction channels. Disruption of the tip link by loud sound or calcium chelators eliminates transduction currents and illustrates that tip-link integrity is critical for mechanosensing. Tip-link remodeling after disruption is a dynamic process, which can lead to the formation of atypical complexes that incorporate alternatively spliced variants of PCDH15. These variants are categorized into six groups (N1–N6) based upon differences in the first two extracellular cadherin (EC) repeats. Here, we characterized the two N-terminal EC repeats of all PCDH15 variants (pcdh15(N1) to pcdh15(N6)) and combined these variants to test complex formation. We solved the crystal structure of a new complex composed of CDH23 EC1-2 (cdh23) and pcdh15(N2) at 2.3 Å resolution and compared it to the canonical cdh23–pcdh15(N1) complex. While there were subtle structural differences, the binding affinity between cdh23 and pcdh15(N2) is ∼6 times weaker than cdh23 and pcdh15(N1) as determined by surface plasmon resonance analysis. Steered molecular dynamics simulations predict that the unbinding force of the cdh23–pcdh15(N2) complex can be lower than the canonical tip link. Our results demonstrate that alternative heterophilic tip-link structures form stable protein–protein interactions in vitro and suggest that homophilic PCDH15–PCDH15 tip links form through the interaction of additional EC repeats.
中文翻译:

通过Protocadherin-15的可变剪接变体来调节内耳尖端连接亲和力。
人类的听力依赖于两种非经典钙黏着蛋白,protocadherin-15(PCDH15)和cadherin-23(CDH23)的尖端相互作用。这些蛋白质一起形成了一条称为尖端的细丝,该细丝连接了机械敏感性毛细胞的相邻立体纤毛。当声波进入耳蜗时,纤毛偏转并向尖端连接施加张力,从而打开附近的传导通道。大声的声音或钙螯合剂破坏了尖端连接,消除了传导电流,并说明了尖端连接的完整性对于机械传感至关重要。破坏后的提示链重塑是一个动态过程,可能导致形成非典型复合物,并结合了PCDH15的可变剪接变体。根据前两个细胞外钙粘着蛋白(EC)重复序列的差异,将这些变体分为六类(N1-N6)。在这里,我们表征了所有PCDH15变体(pcdh15(N1)至pcdh15(N6))的两个N端EC重复序列,并结合了这些变体以测试复合物的形成。我们以2.3的分辨率解析了由CDH23 EC1-2(cdh23)和pcdh15(N2)组成的新复合物的晶体结构,并将其与规范的cdh23–pcdh15(N1)复合物进行了比较。尽管存在细微的结构差异,但通过表面等离子体共振分析确定,cdh23和pcdh15(N2)之间的结合亲和力比cdh23和pcdh15(N1)弱约6倍。操纵分子动力学模拟预测,cdh23–pcdh15(N2)络合物的解键力可能低于规范的尖端链接。在体外,提示同系的PCDH15–PCDH15末端连接是通过其他EC重复序列的相互作用形成的。
更新日期:2018-02-14
中文翻译:

通过Protocadherin-15的可变剪接变体来调节内耳尖端连接亲和力。
人类的听力依赖于两种非经典钙黏着蛋白,protocadherin-15(PCDH15)和cadherin-23(CDH23)的尖端相互作用。这些蛋白质一起形成了一条称为尖端的细丝,该细丝连接了机械敏感性毛细胞的相邻立体纤毛。当声波进入耳蜗时,纤毛偏转并向尖端连接施加张力,从而打开附近的传导通道。大声的声音或钙螯合剂破坏了尖端连接,消除了传导电流,并说明了尖端连接的完整性对于机械传感至关重要。破坏后的提示链重塑是一个动态过程,可能导致形成非典型复合物,并结合了PCDH15的可变剪接变体。根据前两个细胞外钙粘着蛋白(EC)重复序列的差异,将这些变体分为六类(N1-N6)。在这里,我们表征了所有PCDH15变体(pcdh15(N1)至pcdh15(N6))的两个N端EC重复序列,并结合了这些变体以测试复合物的形成。我们以2.3的分辨率解析了由CDH23 EC1-2(cdh23)和pcdh15(N2)组成的新复合物的晶体结构,并将其与规范的cdh23–pcdh15(N1)复合物进行了比较。尽管存在细微的结构差异,但通过表面等离子体共振分析确定,cdh23和pcdh15(N2)之间的结合亲和力比cdh23和pcdh15(N1)弱约6倍。操纵分子动力学模拟预测,cdh23–pcdh15(N2)络合物的解键力可能低于规范的尖端链接。在体外,提示同系的PCDH15–PCDH15末端连接是通过其他EC重复序列的相互作用形成的。


























 京公网安备 11010802027423号
京公网安备 11010802027423号