当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unraveling the Role of a Flexible Tetradentate Ligand in the Aerobic Oxidative Carbon-Carbon Bond Formation with Palladium Complexes: A Computational Mechanistic Study
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-02-14 , DOI: 10.1021/jacs.7b11701 Qian Peng 1 , Zengwei Wang 1 , Snežana D. Zarić 2, 3 , Edward N. Brothers 2 , Michael B. Hall 4
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-02-14 , DOI: 10.1021/jacs.7b11701 Qian Peng 1 , Zengwei Wang 1 , Snežana D. Zarić 2, 3 , Edward N. Brothers 2 , Michael B. Hall 4
Affiliation
|
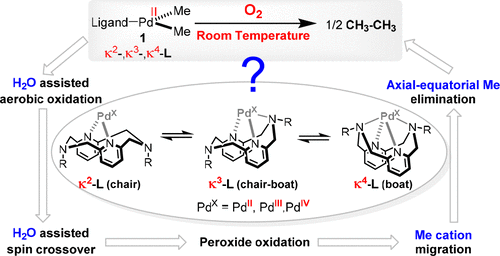
Mechanistic details of the aerobic oxidative coupling of methyl groups by a novel (MeL)PdII(Me)2 complex with the tetradentate ligand, MeL = N, N-dimethyl-2,11-diaza[3.3](2,6)pyridinophane, has been explored by density functional theory calculations. The calculated mechanism sheds light on the role of this ligand's flexibility in several stages of the reaction, especially as the oxidation state of the Pd changes. Ligand flexibility leads to diverse axial coordination modes, and it controls the availability of electrons by modulating the energies of high-lying molecular orbitals, particularly those with major d z2 character. Solvent molecules, particularly water, appear essential in the aerobic oxidation of PdII by lowering the energy of the oxygen molecule's unoccupied molecular orbital and stabilizing the PdX-O2 complex. Ligand flexibility and solvent coordination to oxygen are essential to the required spin-crossover for the transformation of high-valent PdX-O2 complexes. A methyl cation pathway has been predicted by our calculations in transmetalation between PdII and PdIV intermediates to be preferred over methyl radical or methyl anion pathways. Combining an axial and equatorial methyl group is preferred in the reductive elimination pathway where roles are played by the ligand's flexibility and the fluxionality of trimethyl groups.
中文翻译:

揭示柔性四齿配体在钯配合物需氧氧化碳-碳键形成中的作用:计算机制研究
新型 (MeL)PdII(Me)2 配合物与四齿配体 MeL = N, N-二甲基-2,11-二氮杂[3.3](2,6)吡啶烷对甲基有氧氧化偶联的机理细节,已经通过密度泛函理论计算进行了探索。计算出的机制揭示了这种配体的灵活性在反应的几个阶段中的作用,尤其是当 Pd 的氧化态发生变化时。配体的灵活性导致了不同的轴向配位模式,它通过调节高位分子轨道的能量来控制电子的可用性,特别是那些具有主要 d z2 特征的轨道。溶剂分子,尤其是水,通过降低氧分子未占据分子轨道的能量并稳定 PdX-O2 复合物,在 PdII 的有氧氧化中显得必不可少。配体的灵活性和溶剂与氧的配位对于高价 PdX-O2 复合物转化所需的自旋交叉至关重要。通过我们在 PdII 和 PdIV 中间体之间的金属转移中的计算预测,甲基阳离子途径优于甲基自由基或甲基阴离子途径。在还原消除途径中优选结合轴向和赤道甲基,其中配体的灵活性和三甲基的流动性发挥作用。
更新日期:2018-02-14
中文翻译:

揭示柔性四齿配体在钯配合物需氧氧化碳-碳键形成中的作用:计算机制研究
新型 (MeL)PdII(Me)2 配合物与四齿配体 MeL = N, N-二甲基-2,11-二氮杂[3.3](2,6)吡啶烷对甲基有氧氧化偶联的机理细节,已经通过密度泛函理论计算进行了探索。计算出的机制揭示了这种配体的灵活性在反应的几个阶段中的作用,尤其是当 Pd 的氧化态发生变化时。配体的灵活性导致了不同的轴向配位模式,它通过调节高位分子轨道的能量来控制电子的可用性,特别是那些具有主要 d z2 特征的轨道。溶剂分子,尤其是水,通过降低氧分子未占据分子轨道的能量并稳定 PdX-O2 复合物,在 PdII 的有氧氧化中显得必不可少。配体的灵活性和溶剂与氧的配位对于高价 PdX-O2 复合物转化所需的自旋交叉至关重要。通过我们在 PdII 和 PdIV 中间体之间的金属转移中的计算预测,甲基阳离子途径优于甲基自由基或甲基阴离子途径。在还原消除途径中优选结合轴向和赤道甲基,其中配体的灵活性和三甲基的流动性发挥作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号